Method for improving alpha-ketoisocaproate yield through RBS optimization
An amino acid and deaminase technology, applied in the field of genetic engineering, can solve the problems of insufficient industrial production, low yield and conversion rate of α-ketoisocaproic acid, etc., and achieve the effects of easy control, fast reaction rate, and serious pollution problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1R
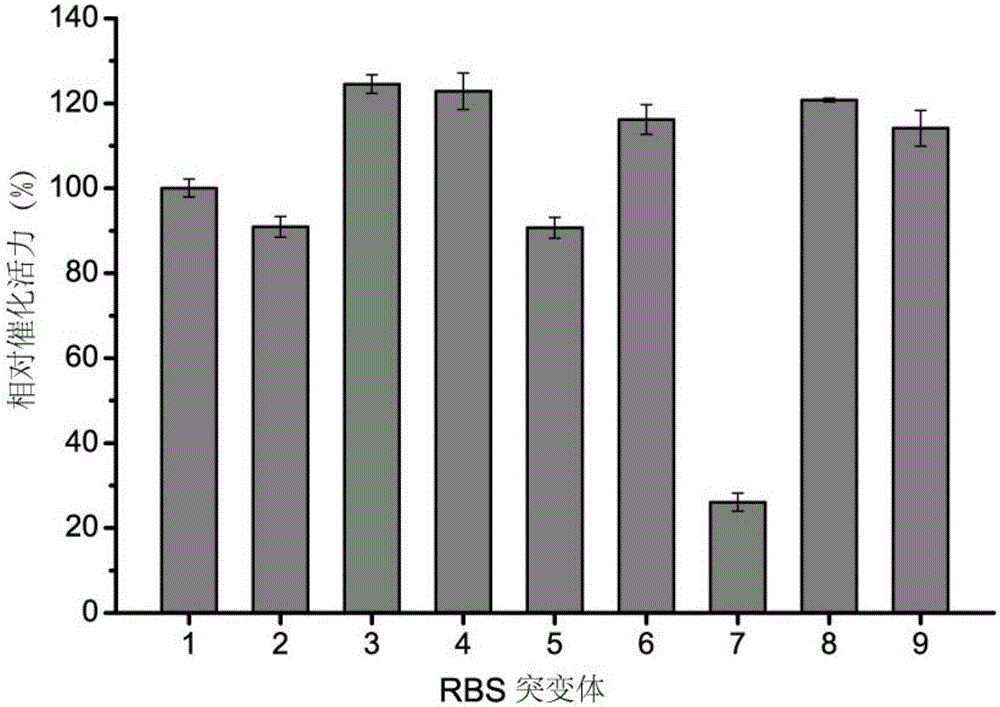
[0022] The construction of embodiment 1RBS mutant library
[0023] Using pET28a-lad (for the construction method, refer to the patent application with application number 201410050399.4 and publication number CN103789247A) as the template, and the forward primer as 5'- NNNNNN ATATACCATGGCGATATCTAGAAGAA-3', the reverse primer is 5'-CTTAAAGTTAAACAAAATTATTTCTAGAGG-3' as the primer for PCR reaction, the reaction product is digested by DpnI after the template is recovered using the DNA column recovery kit, and phosphorylase is added for terminal phosphorylation, adding SolutionI ligase was ligated overnight at 16°C. The ligated plasmid was transformed into the competent E.coliBL21(DE3), and spread on a kanapenicillin-resistant plate.
Embodiment 2
[0024] Example 2 L-amino acid deaminase catalytic activity improved mutant screening
[0025] Use a sterilized toothpick to pick the single colony in Example 1 into a 96-well deep-well plate containing 700 μL of LB medium, cultivate overnight at 37°C with shaking, and inoculate 2% of the inoculum into 96 wells containing 700 μL of fermentation medium In a deep well plate, shake culture at 37°C to OD 600 0.6, add 0.4mMIPTG after 3 hours to collect the bacteria, centrifuge at 3400rpm for 10min, add 1mL of 100mM leucine aqueous solution to the deep well plate, react for 10 minutes, add 100μL of 1% FeCl 3 The solution is colored.
Embodiment 3
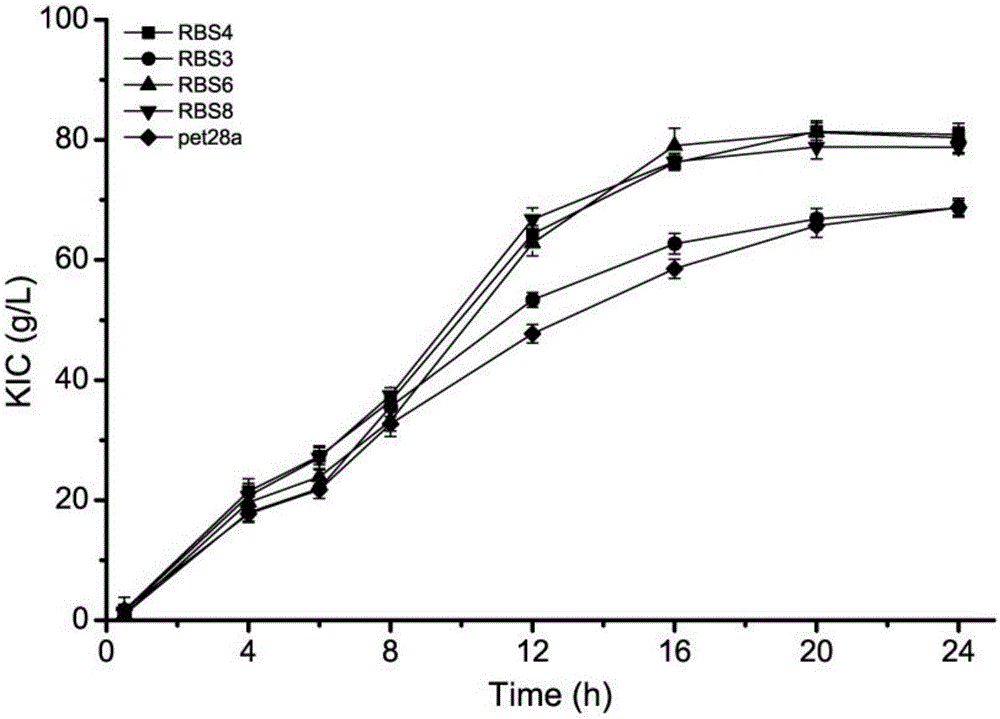
[0026] Example 3 Determination of Catalytic Activity and Sequence Changes of Mutants Converted to L-leucine by Whole Cells
[0027] Recombinant Escherichia coli E.coliBL21 (DE3), which is dark black in Example 2, is transferred from the LB medium to the 250mL Erlenmeyer flask containing 25mL seed medium, cultivated overnight, and inoculated with 2% inoculated into 50mL fermentation medium, cultivated to OD at 37°C, 200r / min 600 0.6, add 0.4mMIPTG to induce, culture at 37°C for 5 hours, and collect the bacteria by centrifugation for whole cell transformation. Take 0.8g / L cells, 100mM substrate L-leucine, react at 37°C for 0.5h, centrifuge at 12000rpm for 2min, and take the supernatant to measure the content of α-ketoisocaproic acid.
[0028] According to the formula Enzyme activity was calculated. (where C (KIC) is the measured α-ketoisocaproic acid content, DCW is 0.8g / L of thalline dry weight, T is the reaction time 0.5h). The catalytic activity of its mutants is as fol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com