Method for recycling rare earth metal from waste fluorescent powder
A technology of rare earth metals and recycling methods, which is applied in the direction of improving process efficiency, etc., and can solve problems affecting continuous operation, dispersion of rare earth metals, and difficult elution of amphoteric metal impurities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
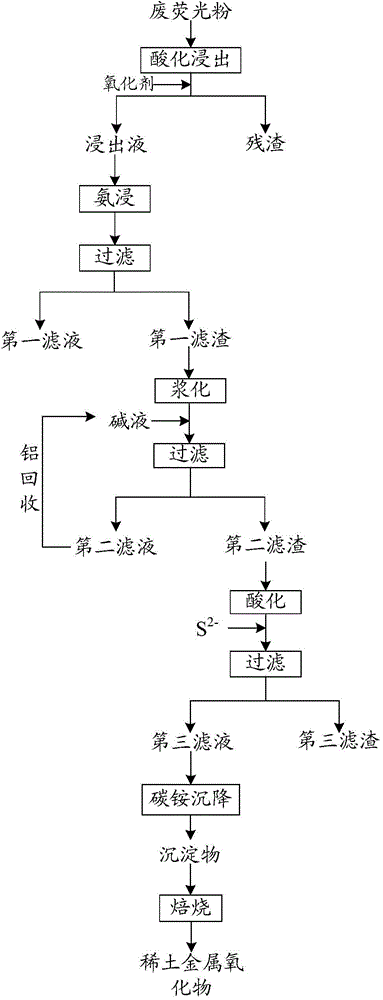
[0045] S10. Add hydrochloric acid with a concentration of 6M to the raw material according to the ratio of solid-liquid volume ratio of 1:6, and stir and dissolve at 75°C; add saturated sodium chlorate solution according to the mass ratio of raw material to sodium chlorate of 1:0.3 . After 3 hours, extract the leaching solution by suction filtration, and test the leaching solution.
[0046] The results are as follows: in the leaching solution, yttrium is 12.81g / L, europium is 854mg / L, zinc is 19.97g / L, aluminum is 482.5mg / L, calcium is 620.5mg / L, lead is 484.0mg / L, and the leaching rate is higher than 99%.
[0047] S20, feed liquid ammonia into the leaching solution until the pH value of the solution is 8, during the process of adding liquid ammonia, the pH value of the solution increases, the rare earth metals and most of the non-rare earth metals form hydroxide precipitates, and the zinc and ammonia form a complex combined to generate soluble zinc ammonium complex ions; an...
Embodiment 2
[0054] S10, add hydrochloric acid with a concentration of 8M to the raw material according to the ratio of solid-liquid volume ratio of 1:8, and stir and dissolve at 60°C; add saturated sodium chlorate solution according to the mass ratio of raw material to sodium chlorate of 1:0.2 . After 2.5 hours, extract the leaching solution by suction filtration, and test the leaching solution.
[0055] The results are as follows: in the leaching solution, yttrium is 11.81g / L, europium is 824mg / L, zinc is 17.92g / L, aluminum is 445.5mg / L, calcium is 672.5mg / L, and lead is 520.0mg / L. The leaching rate is higher than 95%.
[0056] S20. Pass liquid ammonia into the leaching solution until the pH value of the solution is 9, and then filter to obtain a first filtrate and a first filter residue.
[0057] S30, adding 10% sodium hydroxide solution to the first filter residue obtained in S20 to dissolve, and adjusting the pH of the solution to 14, mixing and stirring for 3 hours and filtering to...
Embodiment 3
[0064] Add nitric acid with a concentration of 4M to the raw material according to the solid-liquid volume ratio of 1:5, and stir and dissolve at 85°C; add hydrogen peroxide according to the mass ratio of raw material to hydrogen peroxide at 1:0.4. After 3 hours, extract the leachate by suction filtration.
[0065] S20, feed liquid ammonia into the leaching solution until the pH value of the solution is 10, during the process of adding liquid ammonia, the pH value of the solution rises, the rare earth metals and most of the non-rare earth metals form hydroxide precipitates, and zinc and ammonia form a complex combined to generate soluble zinc ammonium complex ions; and then filtered to obtain the first filtrate and the first filter residue.
[0066] S30, add the first filter residue obtained in S20 to ultrapure water at a weight ratio of 1:2.5 for slurrying, then add 10% sodium hydroxide solution to the slurrying solution and control the pH to 13.5, mix and stir for 2.5 hours ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com