Electrolyte and lithium ion battery using same
An electrolyte and lithium salt technology, applied in secondary batteries, secondary battery repair/maintenance, circuits, etc., can solve difficult problems such as high voltage performance and flame retardancy of electrolytes, achieve good cycle performance and improve safety Performance, flammability reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
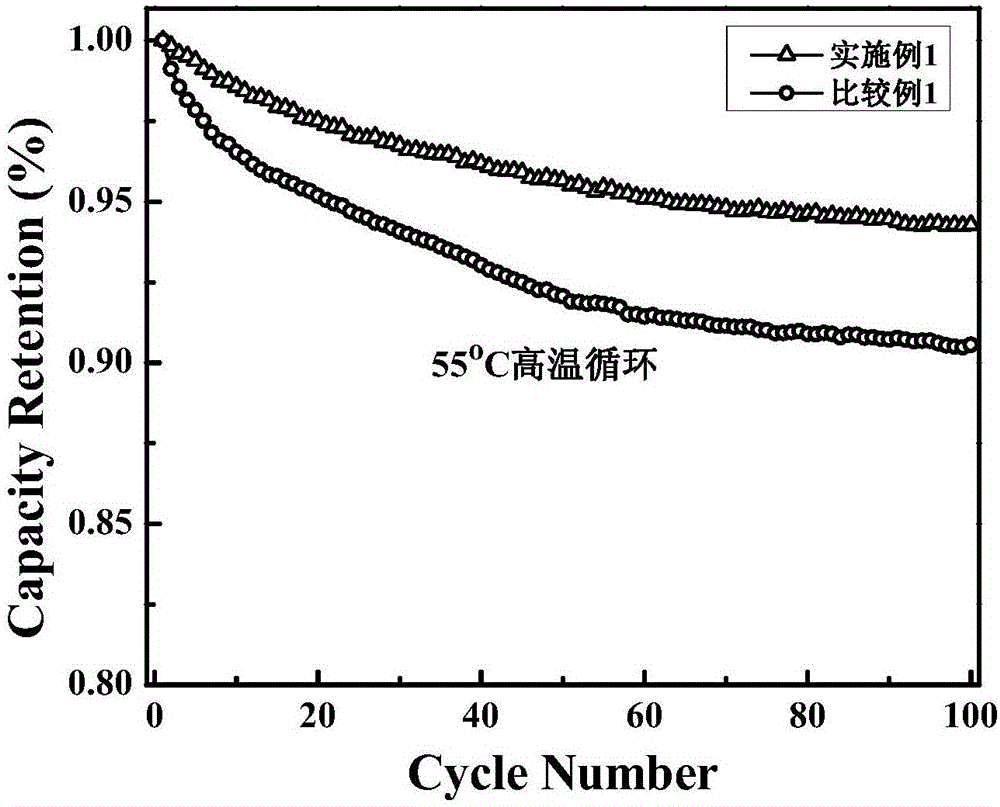
Embodiment 1
[0024] (1) Electrolyte preparation: Prepare 1mol / LLiPF according to EC: PC: DEC: EMC: VC: PS = 35: 5: 35: 25: 2: 2.5 (volume ratio) 6 Electrolyte solution, and then add 1,4 phosphomethyl-2,3 methylcycloalkenyl ester with a weight percent content of 2%.
[0025] (2) Preparation of positive electrode material: LiNi mixed with 91% by weight 0.5 mn 1.5 o 2 (positive active material), the SP (superconducting carbon black) of 4% by weight and the PVDF (binder) of 5% by weight, and add N-methylpyrrolidone to it and make slurry, will The slurry is coated on an aluminum foil, and rolled after drying to obtain the positive electrode material.
[0026] (3) Preparation of negative electrode material: mixing artificial graphite of 75% by weight, mesophase carbon microspheres of 20% by weight, sodium carboxymethyl cellulose of 5% by weight, and adding deionized water, and then coating the slurry on a copper foil, drying and rolling to obtain the negative electrode material.
[0027] (4...
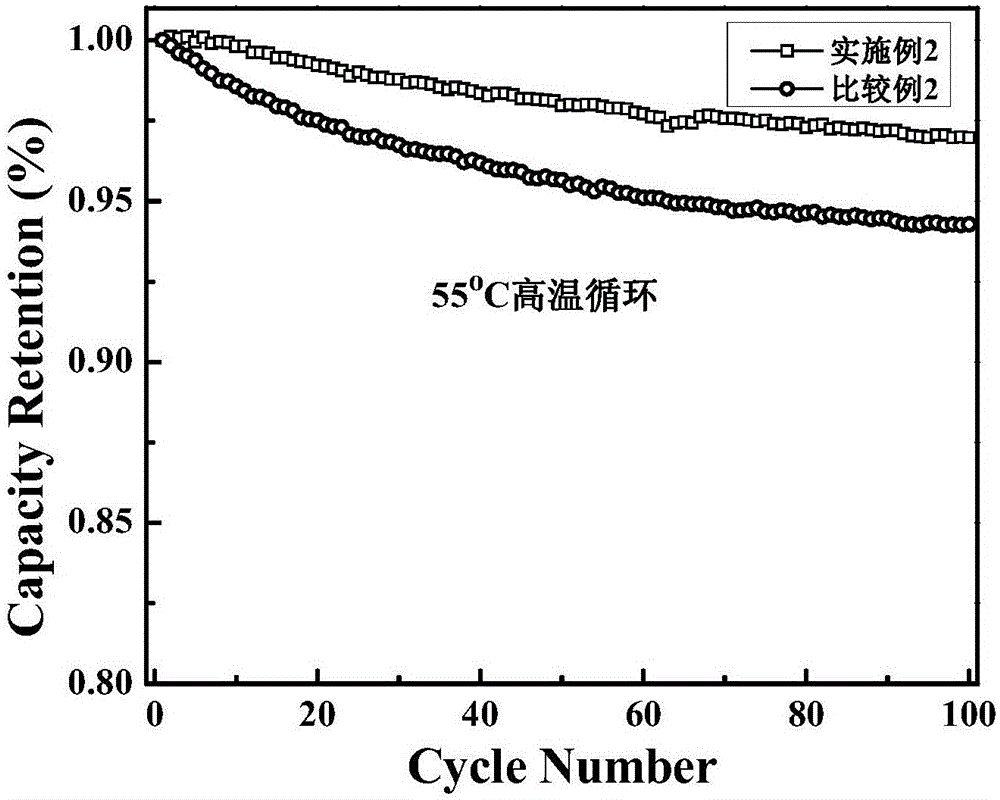
Embodiment 2
[0030] (1) Electrolyte preparation: Prepare 1mol / LLiPF according to EC: PC: DEC: EMC: VC: PS = 35: 5: 35: 25: 2: 2.5 (volume ratio) 6 The electrolytic solution is then added with 2.5% by weight of 1,4-phosphonomethyl-2,3-methylcycloalkenyl ester.
[0031] (2) Preparation of positive electrode material: mixing 72wt% LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2 and 18 wt% LiMn 0.8 Fe 0.2 o 4 (positive electrode active material), the SP (superconducting carbon black) of weight percentage 5% and the PVDF (bonding agent) of weight percentage 5%, and to it, add N-methylpyrrolidone and make slurry, will The slurry is coated on an aluminum foil, and rolled after drying to obtain the positive electrode material.
[0032] (3) Preparation of negative electrode material: mixing artificial graphite of 75% by weight, mesophase carbon microspheres of 20% by weight, sodium carboxymethyl cellulose of 5% by weight, and adding deionized water, and then coating the slurry on a copper foil, drying and rolli...
Embodiment 3
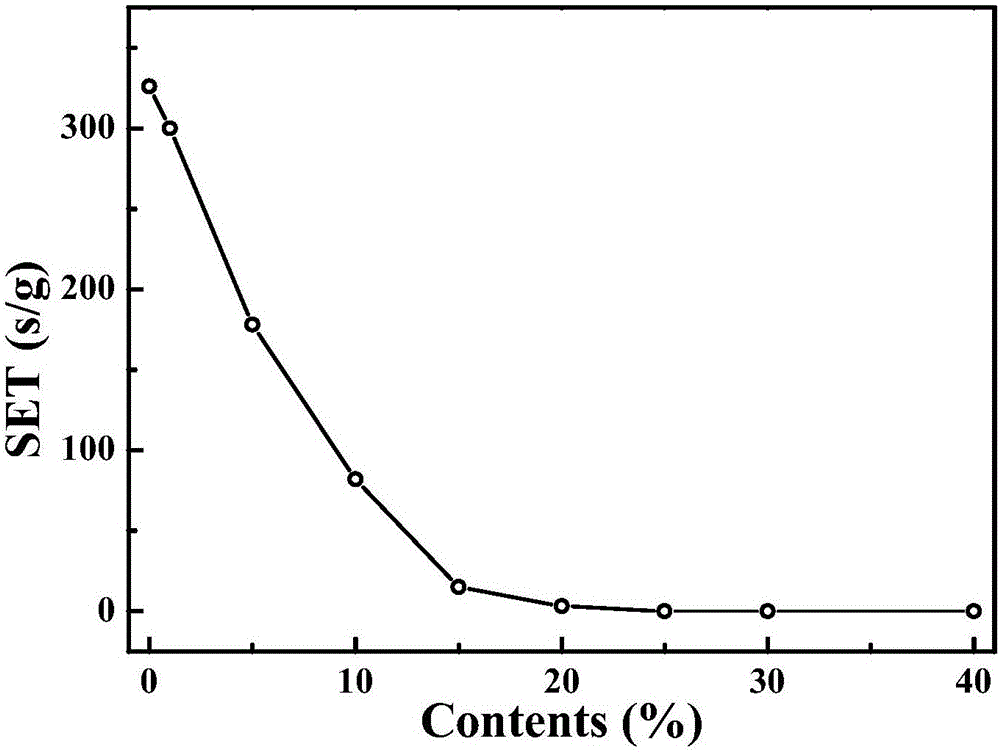
[0036] (1) Configure the reference electrolyte to prepare 1mol / LLiPF according to EC: PC: DEC: EMC: VC: PS = 35: 5: 35: 25: 2: 2.5 (volume ratio) 6 electrolyte, and then 1,4 phosphomethyl-2,3 methylcycloenyl ester was added to the reference electrolyte at different concentrations (1%, 5%, 10%, 15%, 20%, 25%, 30%, 40%), mixed evenly, and the above operations were all completed in an argon glove box.
[0037](2) Use the self-extinguishing time method to test the flame retardancy of the above-mentioned electrolytes containing different concentrations of 1,4-phosphomethyl-2,3 methylcycloenyl ester, and record the test results as follows image 3 It can be obtained that as the content of 1,4-phosphomethyl-2,-methylcycloenyl ester increases, the self-extinguishing time of the electrolyte becomes shorter, and when it is added to 25% or more, the electrolyte is completely non-flammable.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com