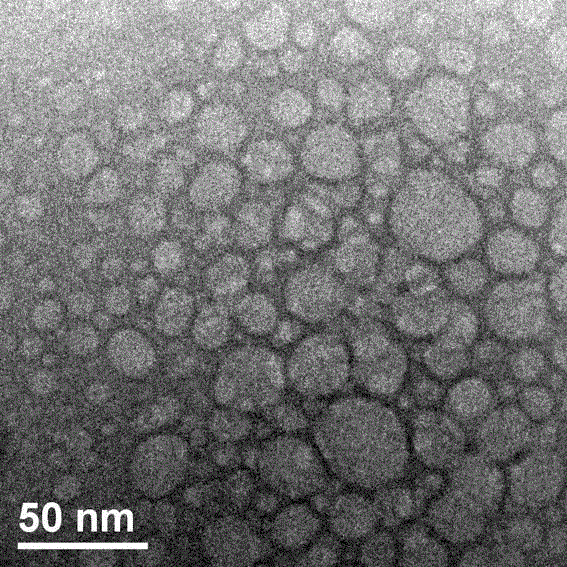
Preparation method of chlorpromazine hydrochloride polycystic sustained-release granules
A technology of chlorpromazine hydrochloride and sustained-release granules, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, capsule delivery, etc. It can solve problems such as high utilization rate, disintegrating drugs, and inconvenience. Achieve the effect of low cost, convenient preparation and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: A preparation method of chlorpromazine hydrochloride multicapsule sustained-release granule, it may further comprise the steps:
[0026] S1. Preparation of the first solution: Sodium hydroxide is added to the phosphate buffer solution in which chlorpromazine hydrochloride is dissolved, and the content of sodium hydroxide in the initial solution is 0.5%; an initial solution is formed, and the copolymer is poured into the initial solution In, the first solution is formed; wherein, the copolymer is a polyoxyethylene-polyoxypropylene-polyoxyethylene triblock copolymer with a polyoxyethylene content of 69%, and the content of the copolymer in the first solution is 65% %; The content of chlorpromazine hydrochloride in the phosphate buffer saline solution that is dissolved with chlorpromazine hydrochloride is 0.8%;
[0027] S2. Preparation of the second solution: dissolving the biodegradable polymer material in an organic solvent to form a second solution; wherein...
Embodiment 2
[0032] Example 2: A preparation method of chlorpromazine hydrochloride multicapsule sustained-release granule, it may further comprise the steps:
[0033] S1. Preparation of the first solution: Add chlorpromazine hydrochloride-dissolved phosphate buffer, and the content of potassium hydroxide in the initial solution is 3%; form the initial solution, pour the copolymer into the initial solution to form The first solution; wherein, the copolymer is a polyoxyethylene-polyoxypropylene-polyoxyethylene triblock copolymer with a polyoxyethylene content of 73%, and the content of the copolymer in the first solution is 75%; The content of chlorpromazine hydrochloride in the phosphate buffer that is dissolved with chlorpromazine hydrochloride is 2.7%;
[0034] S2. Preparation of the second solution: dissolving the biodegradable polymer material in an organic solvent to form a second solution; wherein, the biodegradable polymer material is a poly(lactic-co-glycolic acid) copolymer, and...
Embodiment 3
[0039] Example 3: A preparation method of chlorpromazine hydrochloride multicapsule sustained-release granule, it may further comprise the steps:
[0040] S1. Preparation of the first solution: Add alkali to the phosphate buffer solution that is dissolved with chlorpromazine hydrochloride to form an initial solution, and pour the copolymer into the initial solution to form the first solution; wherein, the copolymer is poly Oxyethylene content is 70% polyoxyethylene-polyoxypropylene-polyoxyethylene triblock copolymer, and the content of copolymer in the first solution is 68%; The alkali is sodium hydroxide and potassium hydroxide, weight Ratio is 1:2, and the content of alkali in initial solution is 1%; The content of chlorpromazine hydrochloride is 1.2% in the described phosphate buffered saline solution that is dissolved with chlorpromazine hydrochloride;
[0041] S2. Preparation of the second solution: dissolving the biodegradable polymer material in an organic solvent to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com