Patents
Literature
33 results about "Chlorpromazine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hydrochloride salt form of chlorpromazine, a phenothiazine and traditional antipsychotic agent with anti-emetic activity. Chlorpromazine hydrochloride exerts its antipsychotic effect by blocking postsynaptic dopamine receptors in cortical and limbic areas of the brain, thereby preventing the excess of dopamine in the brain. This leads to a reduction in psychotic symptoms, such as hallucinations and delusions. Chlorpromazine hydrochloride appears to exert its anti-emetic activity by blocking the dopamine receptors in the chemical trigger zone (CTZ) in the brain, thereby relieving nausea and vomiting.
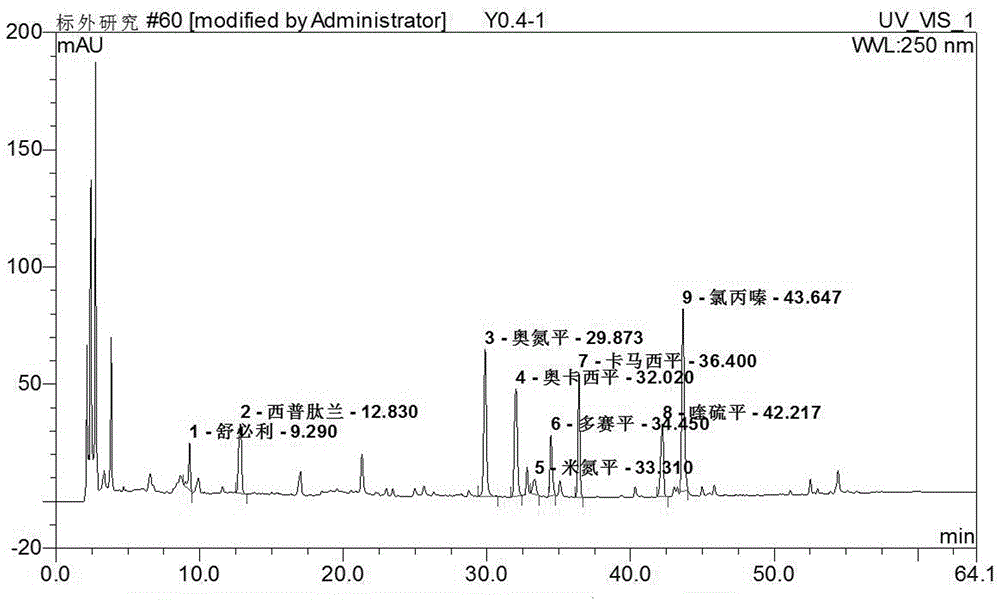
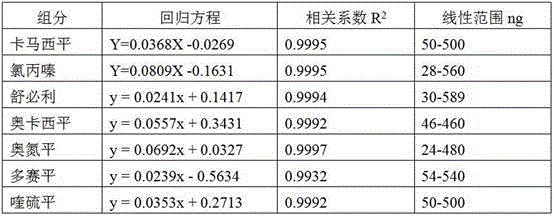
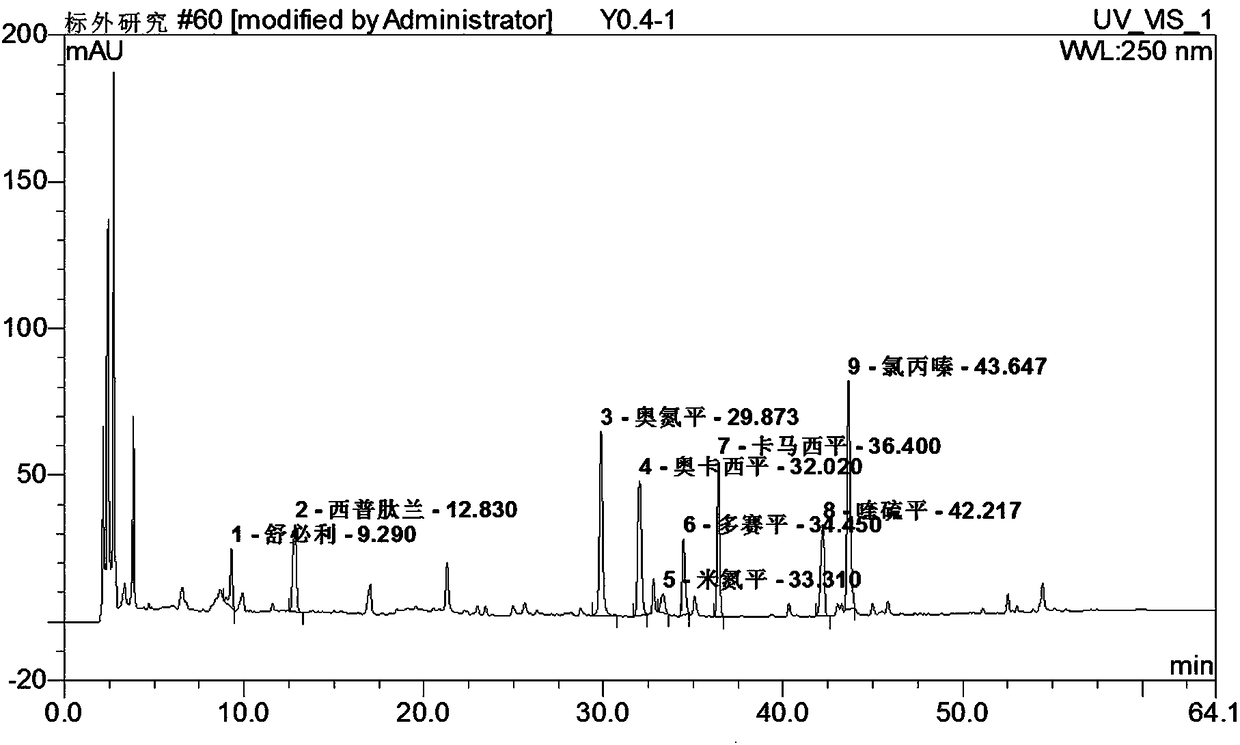
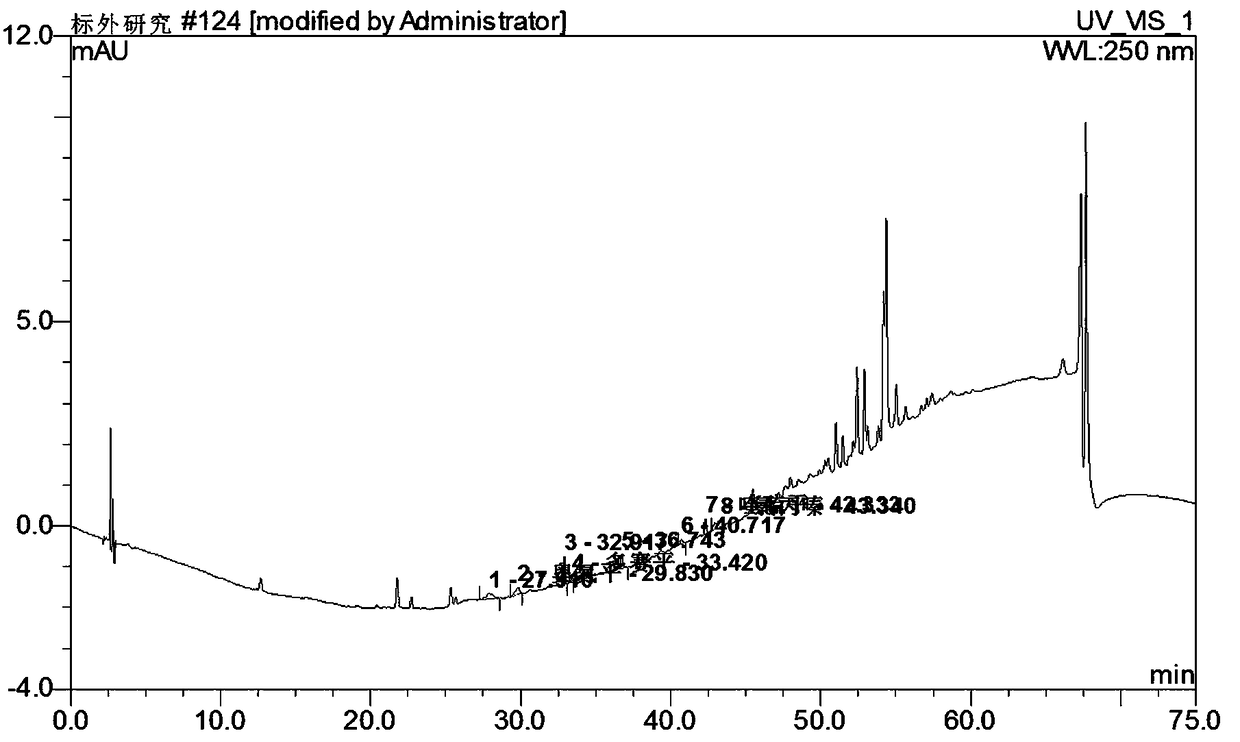
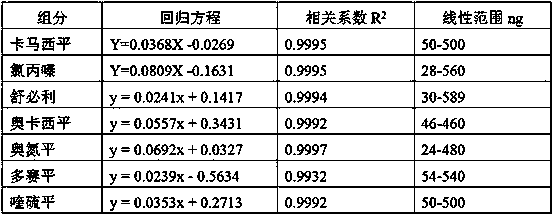
Method for simultaneously detecting seven sleep chemical medicines
The invention discloses a supplemental detection method of seven chemical medicines including carbamazepine, chlorpromazine hydrochloride, olanzapine, doxepin hydrochloride, quetiapine fumarate, oxcarbazepine and sulpiride which are illegally added into health-care food or Chinese patent medicine for improving sleep. After a sample is subjected to ultrasonic extraction with methyl alcohol, chromatogram column separation is conducted, a mobile phase is eluted, and DAN detector is used for detection. By means of a built detection method, methodological verification is conducted, and parameters of results are shown in the description. It is verified that the method is quick, high in specificity and suitable for detection of chemical medicine added to the health-care food or Chinese patent medicine for improving sleep.
Owner:SHANXI PROVINCE FOOD & DRUG INSPECTION INST
Transdermal drug administering system
InactiveCN1663560ALow costReduce wasteMedical devicesPharmaceutical non-active ingredientsVerapamil HydrochlorideWater soluble drug
The invention discloses a percutaneous give drug system, which comprises: a drug storeroom, the gel drug layer comprises drug, solvent, transdermal accelerating agent and gelatinizing agent; one adsorption layer comprised solvent absorption material of solvent in absorbable drug storeroom; one isolating layer between drug storeroom and adsorption layer, which is of non-woven fabrics or semipermeable membrane to make solvent of gas or liquid pass through successfully; adsorption layer can joint isolating layer of drug storeroom in opposite directions of closing up to skin of drug storeroom. The percutaneous give drug system in this invention can increase drug level in drug storeroom of the system to elevate drug transdermal speed, as well as increase controlled release effect of percutaneous give drug and absolute bioavailability, enhance drug curative effect, decrease drug waste, and depress preparation cost of the system. The invention is fit particular to percutaneous give drug system for water-soluble drug of propranolol hydrochloride, verapamil hydrochloride and chlorpromazine hydrochloride.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Method for determining residual sedative type veterinary medicaments in mutton
InactiveCN103954721AMeet Residue Analysis RequirementsHigh recovery rateComponent separationPerphenazinePromethazine
The invention relates to a method for determining residual medicaments in mutton, and in particular relates to a method for determining multiple sedative type medicaments in mutton at the same time. The residual sedative type veterinary medicaments refer to zolpidem, haloperidol, chlordiazepoxide, promethazine, nitrazepam, chlorpromazine hydrochloride, perphenazine, fluphenazine hydrochloride, clonazepam, xylazine hydrochloride, propionylpromazine, carazolol, acepromazine, droperidol and azaperone. The method has the advantage that the residual amounts of 15 types of sedative type medicaments in mutton are determined through high-resolution liquid chromatography-tandem mass spectrometry. The method is high in sensitivity and high in recovery rate, and can meet the detection requirements on veterinary medicaments.
Owner:GANSU AGRI UNIV
Method for improving fermentation yield of long-chain dicarboxylic acid
InactiveCN102808004AReduce unit consumptionImprove conversion rateMicroorganism based processesFermentationAlkaneBeta oxidation
The invention belongs to the technical field of chemical products, and relates to a method for improving the fermentation yield of long-chain dicarboxylic acid. According to the method, the long-chain dicarboxylic acid is produced by a microbiological fermentation method by taking C10 to C18 n-alkanes as raw materials; the method is characterized in that alpha oxidative decarboxylation inhibitor and beta-oxidation inhibitor are added in a fermentation production process to reduce the alpha-oxidative decarboxylation and beta-oxidation capacities of strains, wherein the alpha oxidative decarboxylation inhibitor is one or mixture of more of chlorpromazine hydrochloride, phenobarbital sodium, crylic acid and polyacrylic acid, and the concentration is 0.01 to 1mmol / L; and the beta-oxidation inhibitor is one or mixture of more of mercaptoacetic acid, sodium thioglycollate, ranolazine, ranolazine dihydrochloride and mildronate, and the concentration is 0.01 to 1 mmol / L. Compared with the prior art, the method has the advantages of high acid producing speed, weak alpha oxidative decarboxylation and beta-oxidation capacity, low unit consumption of alkane, and high fermentation alkane conversion rate, and is particularly suitable for preparing the long-chain dicarboxylic acid.
Owner:ZIBO GUANGTONG CHEM
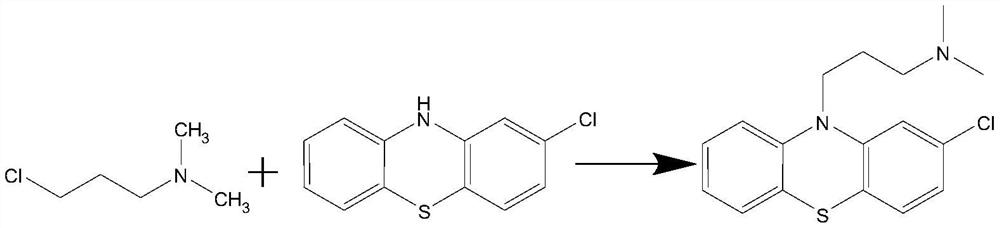
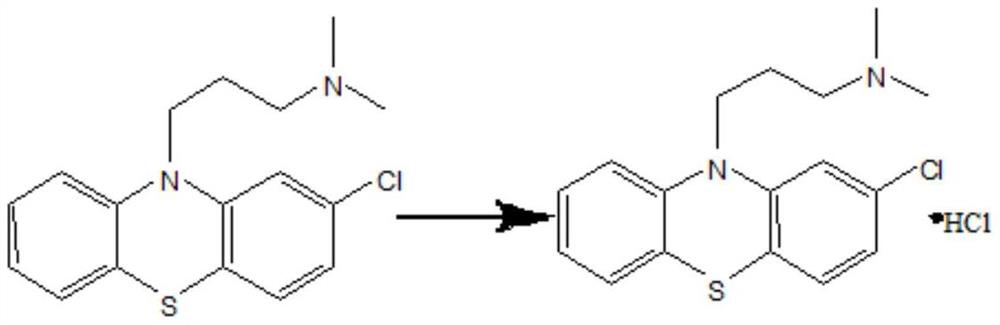
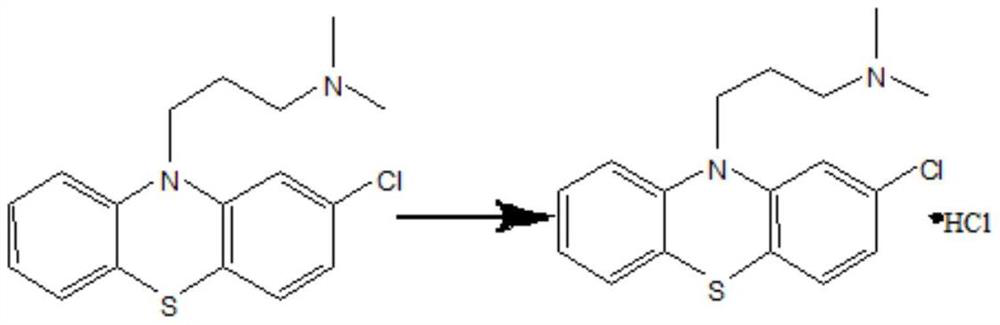
Chlorpromazine hydrochloride synthesis process
The invention relates to a chlorpromazine hydrochloride synthesis process, which includes the steps: leading 2-chlorophenothiazine of a primary ring and a side chain of N, N-dimethyl-3-chloropropylamine, which serve as raw materials, to react with sodium hydroxide and tetrabutylammonium bromide, which serve as condensing agent, to generate chlorpromazine; and salifying the chlorpromazine and hydrogen chloride so that chlorpromazine hydrochloride is synthesized. By means of selecting the sodium hydroxide and the tetrabutylammonium bromide as the condensing agent, and strictly controlling the raw material ratio and an indicator end point of salifying reaction, molar yield of the chlorpromazine can be higher than 90%, and the quality of the chlorpromazine hydrochloride meets the requirements of the BP (British Pharmacopoeia).
Owner:常州康普药业有限公司
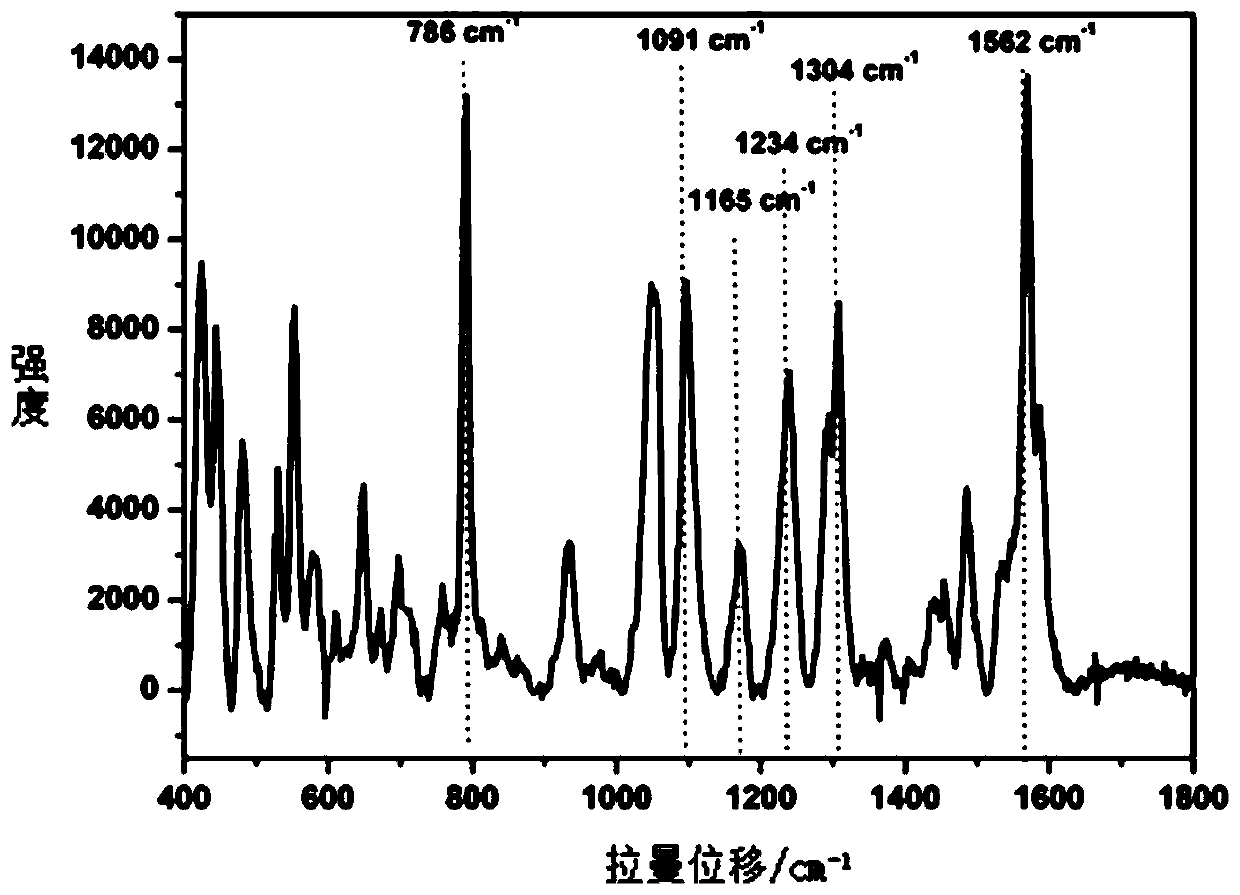
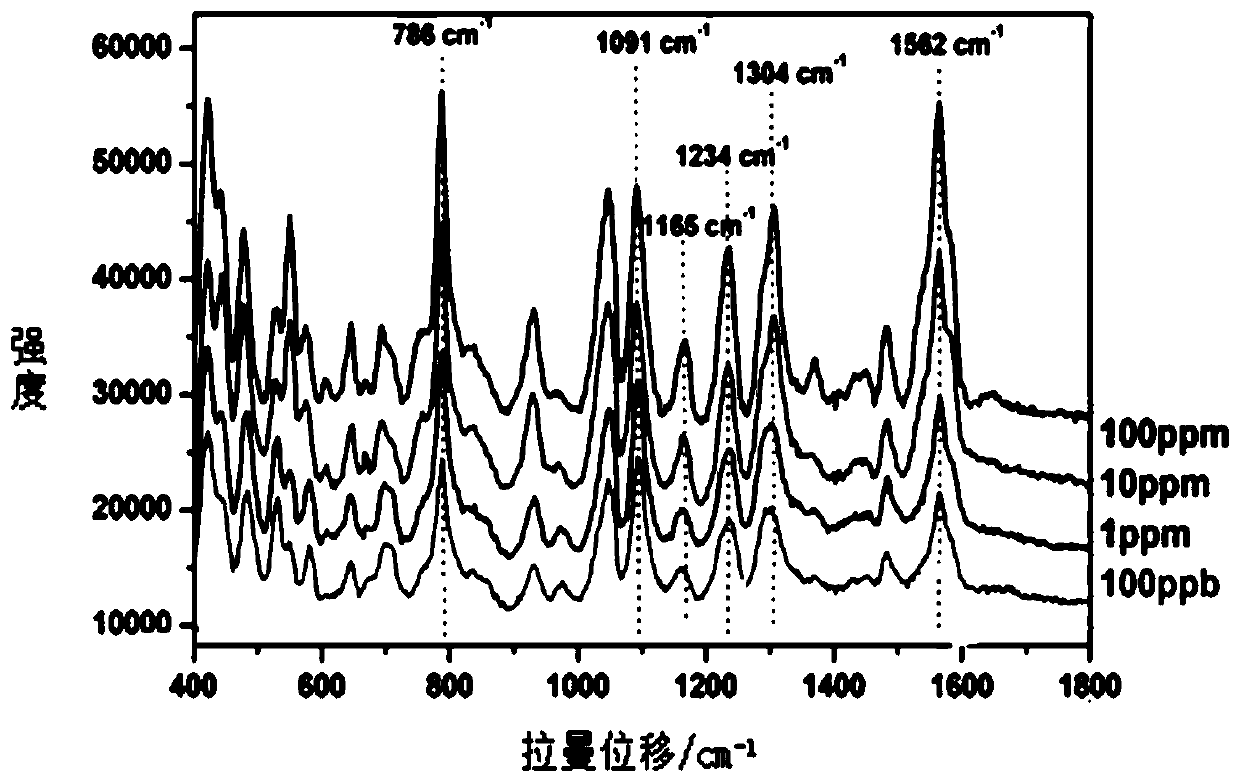
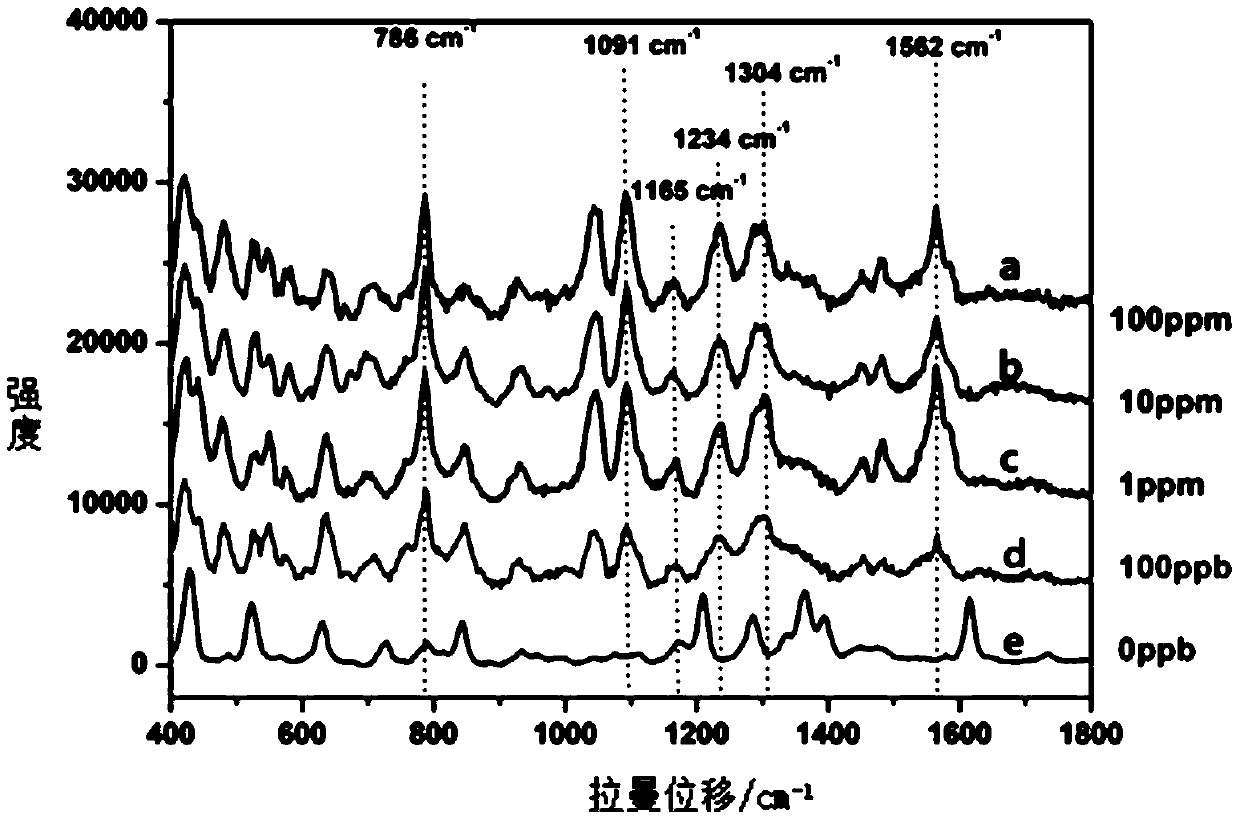
Method of quickly detecting chlorpromazine hydrochloride in fodder
The invention discloses a method of quickly detecting chlorpromazine hydrochloride in fodder. The method comprises the steps that (1) a sample is extracted, and a to-be-detected liquid is obtained; (2) the to-be-detected liquid is added into a surface enhancer, then a coagulant is added, and a mixed liquid is obtained after uniform mixing; (3) a Raman spectrometer is adopted to perform Raman spectrum determination on a blank sample containing a standard substance, and a chlorpromazine hydrochloride standard substance database is established; (4) the Raman spectrometer is adopted to perform Raman spectrum determination on the mixed liquid; and (5) the Raman spectrometer automatically recognizes a negative / positive sample. Through the method, a pretreatment step in an analysis process can besimplified, the detection cycle can be shortened, the detection sensitivity can be improved, and the detection cost can be lowered.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
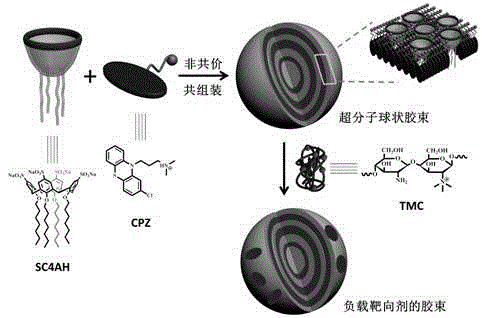
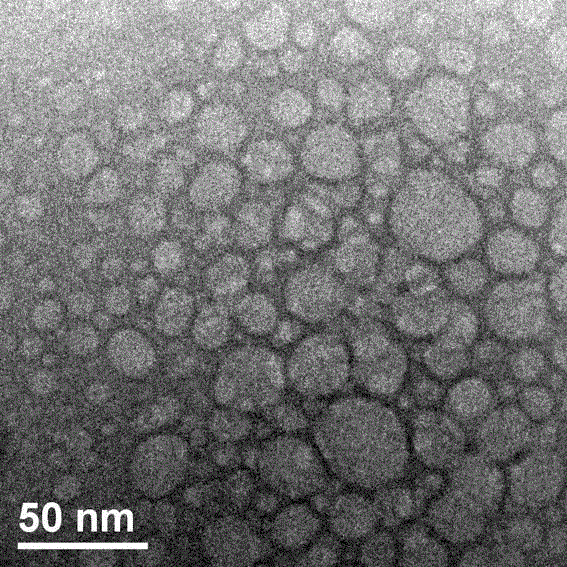
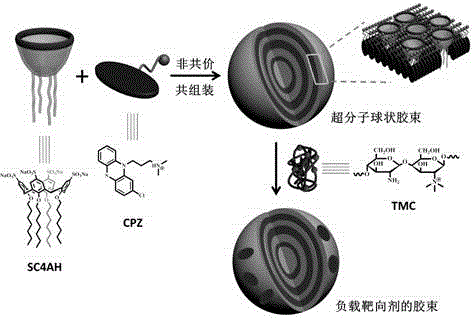
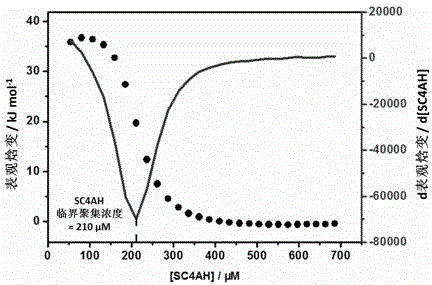
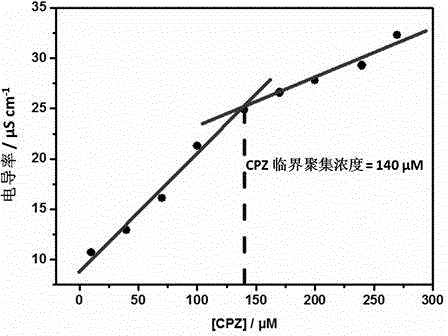
Preparation method of supermolecule globular micelle based on antidepressant medicament chlorpromazine
InactiveCN103550156AReduce dosageHigh load rateOrganic active ingredientsNervous disorderDrug carrierTrimethyl chitosan
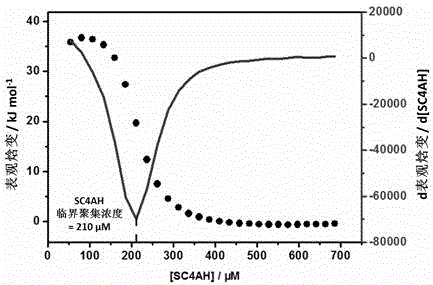
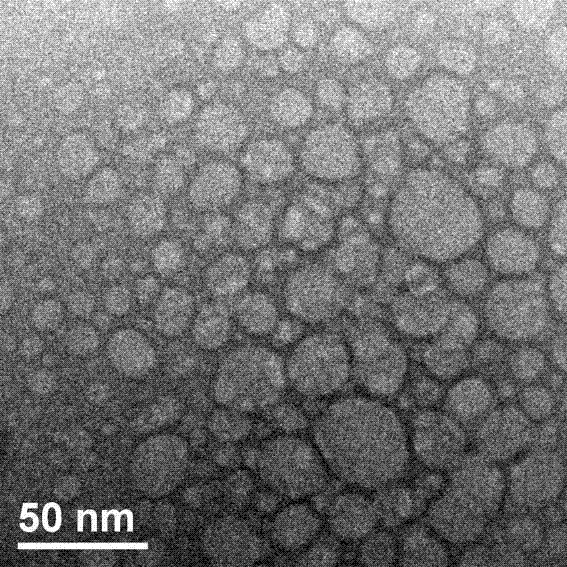
The invention discloses a preparation method of a supermolecule globular micelle based on an antidepressant medicament chlorpromazine. The method comprises the following steps: constructing a medicament carrier through the supermolecule interaction of amphiphilic sulfonated calix [4] arene and an antidepressant medicament chlorpromazine hydrochloride, dissolving the amphiphilic sulfonated calix [4] arene and the antidepressant medicament chlorpromazine hydrochloride in water, and uniformly mixing to obtain a nano supermolecule globular micelle solution; and non-covalently modifying a targeting agent trimethyl chitosan through a bonding site of the calix arene on the surface of the micelle to obtain the supermolecule micelle loaded with trimethyl chitosan on the surface. The preparation method has the advantages that the preparation method is simple and convenient, and fewer subjective and objective raw materials are used; the medicament load rate is high since the medicament molecules are the constructing units of the medicament carrier and the medicament molecules are immobilized; the micelle can be used for non-covalently modifying the targeting agent trimethyl chitosan through the bonding site of the surface calix arene so that the micelle has a wide application prospect in the field of targeted medicament delivery.
Owner:NANKAI UNIV
Transdermal drug administering system
InactiveCN100386073CLow costReduce wasteMedical devicesPharmaceutical non-active ingredientsVerapamil HydrochlorideWater soluble drug
The invention discloses a percutaneous give drug system, which comprises: a drug storeroom, the gel drug layer comprises drug, solvent, transdermal accelerating agent and gelatinizing agent; one adsorption layer comprised solvent absorption material of solvent in absorbable drug storeroom; one isolating layer between drug storeroom and adsorption layer, which is of non-woven fabrics or semipermeable membrane to make solvent of gas or liquid pass through successfully; adsorption layer can joint isolating layer of drug storeroom in opposite directions of closing up to skin of drug storeroom. The percutaneous give drug system in this invention can increase drug level in drug storeroom of the system to elevate drug transdermal speed, as well as increase controlled release effect of percutaneous give drug and absolute bioavailability, enhance drug curative effect, decrease drug waste, and depress preparation cost of the system. The invention is fit particular to percutaneous give drug system for water-soluble drug of propranolol hydrochloride, verapamil hydrochloride and chlorpromazine hydrochloride.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Bait II used in shrimp-catching square basket or cage in summer
InactiveCN102160540AIncrease trappingImprove timelinessOther angling devicesShrimpVitamin B6 synthesis
The invention relates to a bait II used in a shrimp-catching square basket or cage in summer, and the bait II is prepared from the following raw materials by weight: 300 to 700 g of small crucian carp, 750 g of wheat flour, 80 to 120 g of fresh celery, 100 tablets of vitamin B6 slow-release tablet (50 mg / tablet), 100 tablets of chlorpromazine hydrochloride tablet (25 mg / tablet), and 80 g of water. The bait is prepared through the following steps: chopping small crucian carps and fresh celery respectively into pastes, mixing the pastes together with wheat flour and water, stirring thoroughly, making columns with the diameter of 1-2 cm, cooking in a pot with water, and taking out from the pot; cutting to segments with a length of about 1 to 2 cm, and embedding one vitamin B6 slow-release tablet (1) and one chlorpromazine hydrochloride tablet (2) at the two ends of the mixture respectively; and finally placing the segments in the shrimp-catching square basket or cage, fixing the square basket or cage on a baiting rod, and putting in water for shrimp catching. The bait has the advantages that the shrimp-entrapping capacity is increased and the fabrication is easy.
Owner:李万民
Chlorpromazine hydrochloride synthesis process
The invention relates to a chlorpromazine hydrochloride synthesis process, which includes the steps: leading 2-chlorophenothiazine of a primary ring and a side chain of N, N-dimethyl-3-chloropropylamine, which serve as raw materials, to react with sodium hydroxide and tetrabutylammonium bromide, which serve as condensing agent, to generate chlorpromazine; and salifying the chlorpromazine and hydrogen chloride so that chlorpromazine hydrochloride is synthesized. By means of selecting the sodium hydroxide and the tetrabutylammonium bromide as the condensing agent, and strictly controlling the raw material ratio and an indicator end point of salifying reaction, molar yield of the chlorpromazine can be higher than 90%, and the quality of the chlorpromazine hydrochloride meets the requirements of the BP (British Pharmacopoeia).
Owner:常州康普药业有限公司
Chlorpromazine hydrochloride chewable tablets for dog or cat
InactiveCN101190199ABreak through the shortcomings of poor palatabilityHigh cure rateOrganic active ingredientsAnaestheticsOral glucoseDisease
The invention discloses a chlorpromazine hydrochloride chewable tablet used for dogs and cats. The invention can overcome the disadvantage of the poor palatability of the existing tablets, promote dogs and cats to chew and adsorb fully, effectively ensure the dosage of administration, enhance the cure rate of feline or canine diseases and reduce the waste of medicine. The tablet of the invention includes the following components represented by weight-percentage respectively: 1-5 percent of aspartame, 20-30 percent of the mixture of oral glucose and skim milk powder, in which the ratio of oral glucose and skim milk powder is 1:1-1:4, 40-60 percent of excipient, 0.5-1 percent of glidant and 5-20 percent of chlorpromazine hydrochloride.
Owner:TIANJIN RINGPU BIO TECH
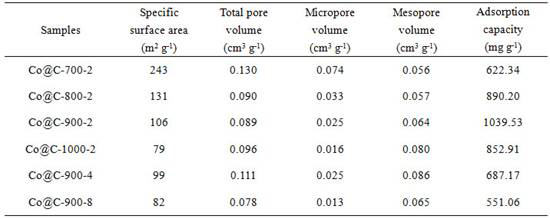
Preparation method and application of cobalt-based metal organic framework derived magnetic carbon composite material
ActiveCN113786805AImprove magnetic propertiesEasy to prepareOther chemical processesSpecific water treatment objectivesMagnetic carbonSorbent
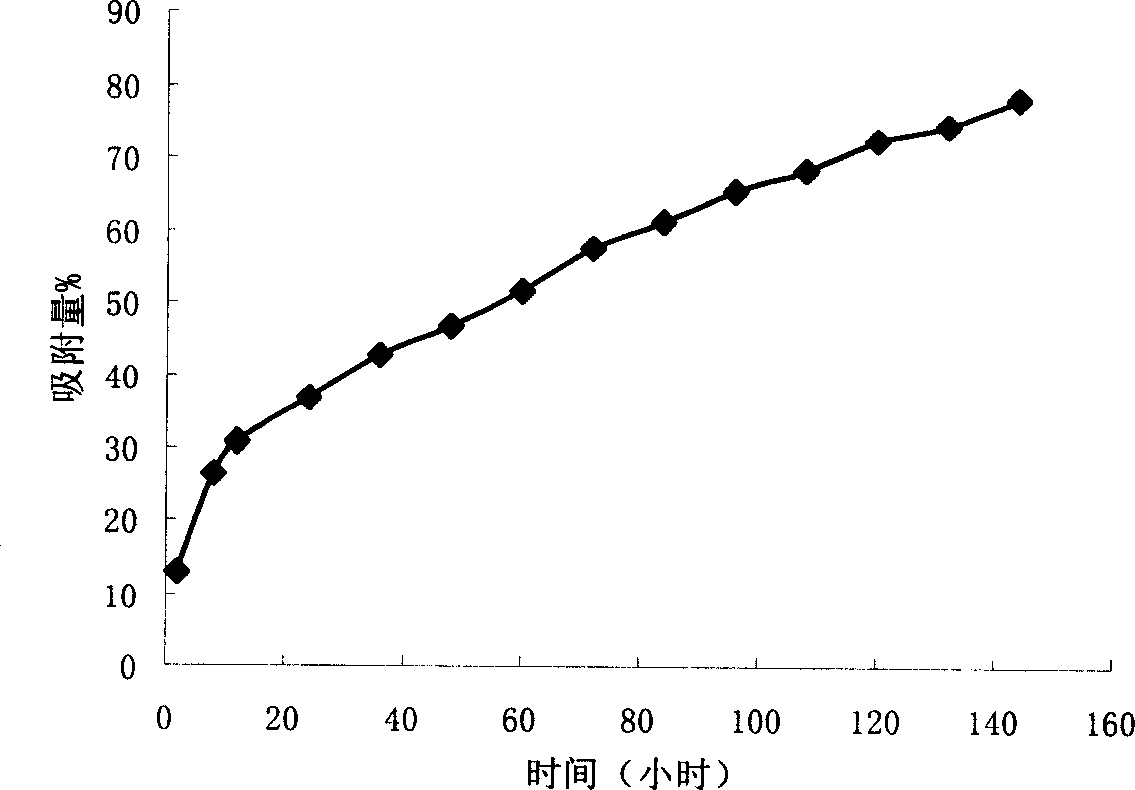
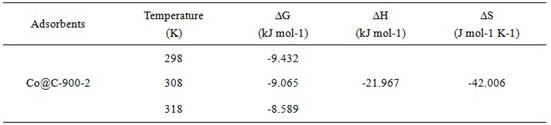
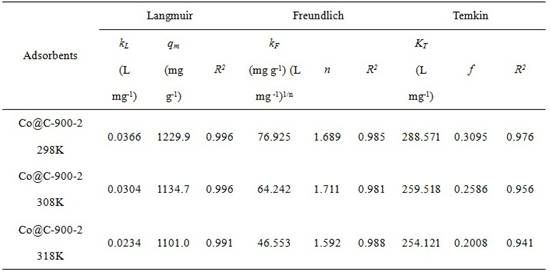
The invention discloses a preparation method and application of a cobalt-based metal organic framework derived magnetic carbon composite material. The preparation method comprises the following steps: 1) preparing a cobalt-based metal organic framework compound Co-MONCs; and 2) performing high-temperature carbonization on the cobalt-based metal organic framework compound Co-MONCs to generate a cobalt-based metal organic framework derived magnetic carbon composite material; wherein the cobalt-based metal organic framework derived magnetic carbon composite material prepared by the preparation method is used as a drug pollutant adsorbent for adsorbing chlorpromazine hydrochloride in a water body. The preparation method disclosed by the invention is simple, sufficient in raw material source, low in production cost, suitable for expanded production requirements and convenient for industrial production; and the cobalt-based metal organic framework derived magnetic carbon composite material prepared by the preparation method has very good adsorption capacity on chlorpromazine hydrochloride, has good desorption capacity and cyclic utilization capacity, also has magnetism, can be quickly recovered through a magnet, and is convenient to recover.
Owner:GUANGDONG MEDICAL UNIV
Medicine for treating dysmenorrhea
InactiveCN106729604AMeeting urgent needsLittle side effectsOrganic active ingredientsHydrolysed protein ingredientsSide effectDiclofenac Sodium
The invention discloses a pharmaceutical composition for treating dysmenorrhea. The pharmaceutical composition comprises the following raw materials in parts by weight: 2-5 parts of herba leonuri, 2-5 parts of safflower carthamus, 0.1-2 parts of the rhizome of chuanxiong, 0.8-5 parts of EGCG, 1-5 parts of citric acid, 0.1-1 part of chlordiazepoxide, 5-10 parts of olive oil, 2-5 parts of chlorpromazine hydrochloride, 0.2-1 part of vitamin p, 1-5 parts of an earthworm protein powder, 5-9 parts of colla corii asini, 1-5 parts of diclofenac sodium, 2-8 parts of vitamin E, 2-5 parts of glucocorticoid drugs and 80-150 parts of normal saline. The raw materials are compounded to take a synergistic effect, and the pharmaceutical composition can effectively treat symptoms, such as stomachache, waist soreness and weakness and hand and foot coldness in menstruation, and is simple to prepare and low in production cost. The medicine can radically cure dysmenorrhea, prevents patients from drug dependence, is small in side effect and avoids complications.
Owner:HENAN SHUIJINGTOU CULTURAL MEDIA CO LTD
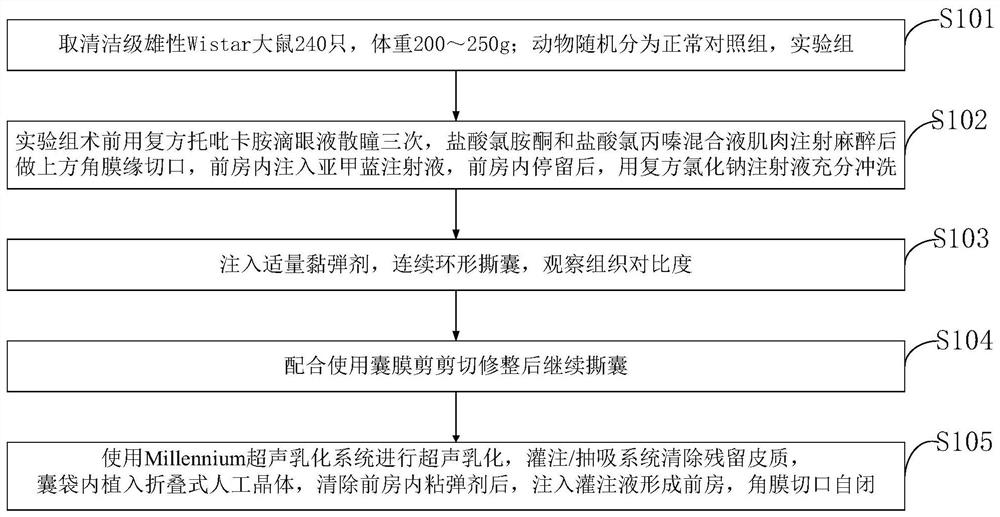
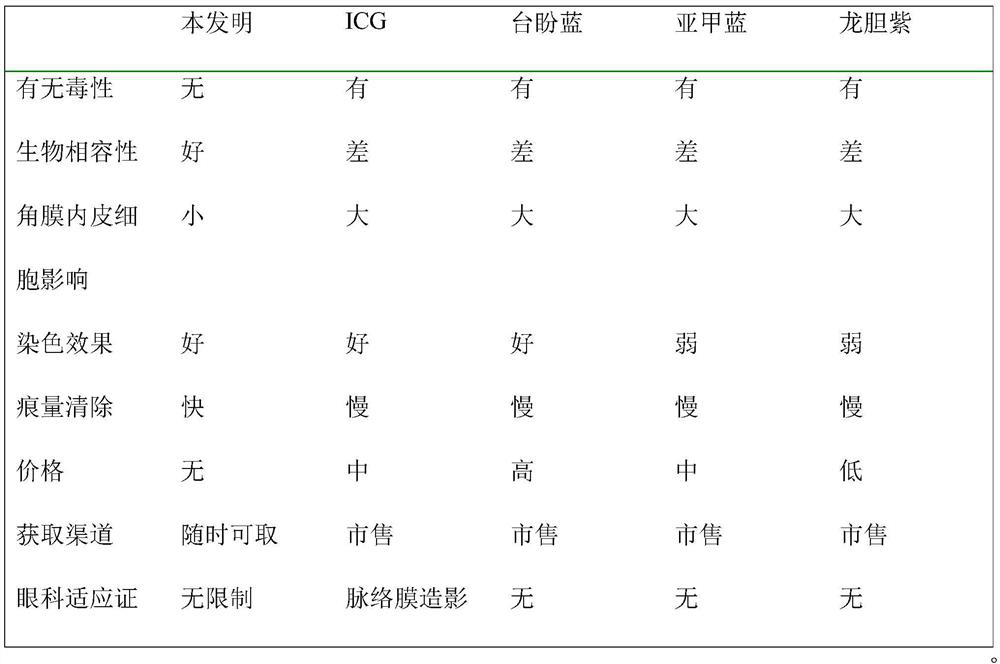
Lens anterior capsule staining method for hypermature cataract surgery
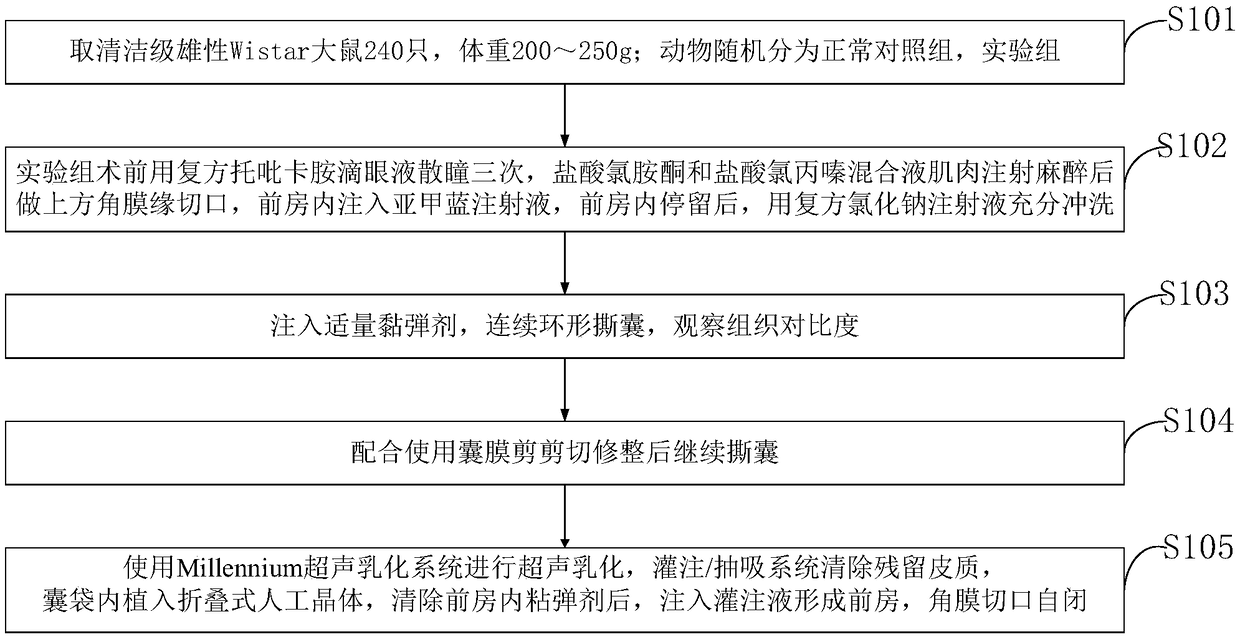
ActiveCN109481141ANo drug chemical toxicityNo complicationsEye surgerySurgical veterinaryStainingBiocompatibility Testing
The invention belongs to the technical field of medicine and discloses a lens anterior capsule staining method for hypermature cataract surgery. 240 Clean-level male Wistar rats weighing 200-250 g aretaken; the rats are randomly divided into a normal control group and an experiment group; compound tropicamide eye drops are used to dilate pupils of the experiment group three times before surgery,muscular injection is performed with ketamine hydrochloride and chlorpromazine hydrochloride mixture for anesthesia before superior corneal limbus incising is performed, the anterior chamber is injected with methylthioninium chloride injection, and after the injection stays in the anterior chamber, full irrigating is performed with compound sodium chloride injection; suitable viscoelastic agent isinjected, and continuous circular capsulorhexis is performed to observe tissue contrast; capsulotomy vannas scissors are used to perform cutting and trimming before capsulorhexis is continued. Additional preparations are not required herein; using is available at any time; biocompatibility is good; no pharmaceutical chemical toxicity is caused to intraocular tissues, such as corneal endothelial cells and visual cells; residual trace can be discharged by means normal intraocular absorption or aqueous circulation; no evident complications occur.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Processing technology of chlorpromazine tablet
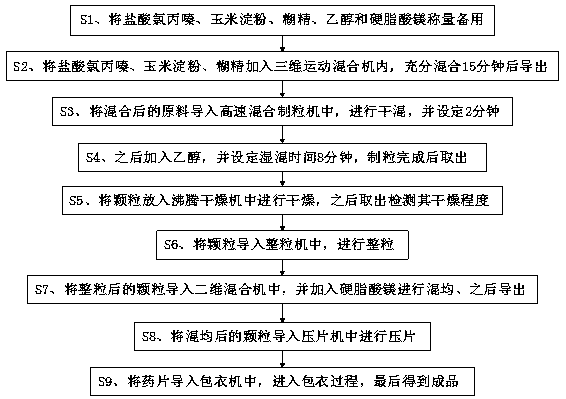
InactiveCN110638766AEasy to processLower manufacturing requirementsOrganic active ingredientsNervous disorderMagnesium stearateStearic acid
The invention relates to the technical field of drugs, and discloses a processing technology of a chlorpromazine tablet. The chlorpromazine tablet is prepared from the following crude drugs in parts by weight: 25 parts of chlorpromazine hydrochloride, 15 parts of corn starch, 6 parts of dextrin, 20 parts of ethanol and 2 parts of magnesium stearate. The chlorpromazine tablet is processed through the following specific processing steps: the chlorpromazine hydrochloride, the corn starch, the dextrin, the ethanol and the magnesium stearate are weighed for standby application; and the chlorpromazine hydrochloride, the corn starch and the dextrin are added into a three-dimensional motion mixer to be fully mixed for 15 minutes and then guided out. According to the processing technology of the chlorpromazine tablet, the current processing process of the chlorpromazine tablet is simplified, the tedious processing steps are simplified, the processing cost is reduced, and processing is convenient; and although the technology is simplified, the standard for the technology is not lowered, so that the quality of drugs is well ensured, and through the technology, the preparation requirements ofthe chlorpromazine tablet are lowered so as to facilitate preparation in many small and medium-sized pharmaceutical factories.
Owner:HUAYI PHARMA ANHUI CO LTD
Preparation method of chlorpromazine hydrochloride polycystic sustained-release granules
ActiveCN105125523ARelease stabilityUniform sizeOrganic active ingredientsNervous disorderFreeze-dryingPharmaceutical drug
The invention discloses a preparation method of chlorpromazine hydrochloride polycystic sustained-release granules. The method includes the steps of S1, preparing first solution; S2, preparing second solution; S3, preparing third solution; S4, preparing final solution; S5, stirring and drawing pressure; S6 freeze drying. The preparation method has the advantages that the sustained-release granules are prepared by using degradable polymer materials to entrap chlorpromazine, medicine is wrapped in the sustained-release granules to form the polycystic granules even in granule size, the medicine is released in a sustained manner, disintegration, causing medicine burst release, of the granules is avoided after the granules are degraded in vivo for a certain time, and the method is simple to operate, convenient in preparation, low in cost and suitable for large-scale industrial production.
Owner:嘉兴天源药业有限公司
Preparation method of supermolecule globular micelle based on antidepressant medicament chlorpromazine
InactiveCN103550156BReduce dosageHigh load rateOrganic active ingredientsNervous disorderDrug carrierTrimethyl chitosan
The invention discloses a preparation method of a supermolecule globular micelle based on an antidepressant medicament chlorpromazine. The method comprises the following steps: constructing a medicament carrier through the supermolecule interaction of amphiphilic sulfonated calix [4] arene and an antidepressant medicament chlorpromazine hydrochloride, dissolving the amphiphilic sulfonated calix [4] arene and the antidepressant medicament chlorpromazine hydrochloride in water, and uniformly mixing to obtain a nano supermolecule globular micelle solution; and non-covalently modifying a targeting agent trimethyl chitosan through a bonding site of the calix arene on the surface of the micelle to obtain the supermolecule micelle loaded with trimethyl chitosan on the surface. The preparation method has the advantages that the preparation method is simple and convenient, and fewer subjective and objective raw materials are used; the medicament load rate is high since the medicament molecules are the constructing units of the medicament carrier and the medicament molecules are immobilized; the micelle can be used for non-covalently modifying the targeting agent trimethyl chitosan through the bonding site of the surface calix arene so that the micelle has a wide application prospect in the field of targeted medicament delivery.
Owner:NANKAI UNIV
Application of chlorpromazine hydrochloride in treatment of endometrial cancer
PendingCN114588163AOrganic active ingredientsAntineoplastic agentsEndometrial CarcinomasPhosphorylation
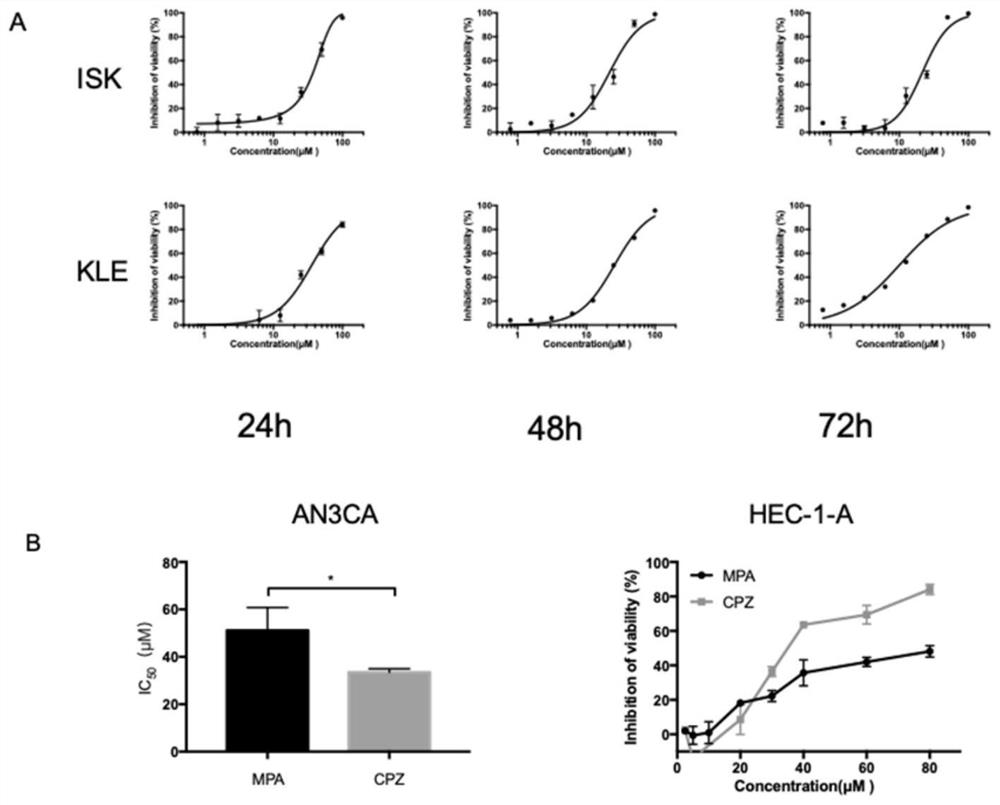
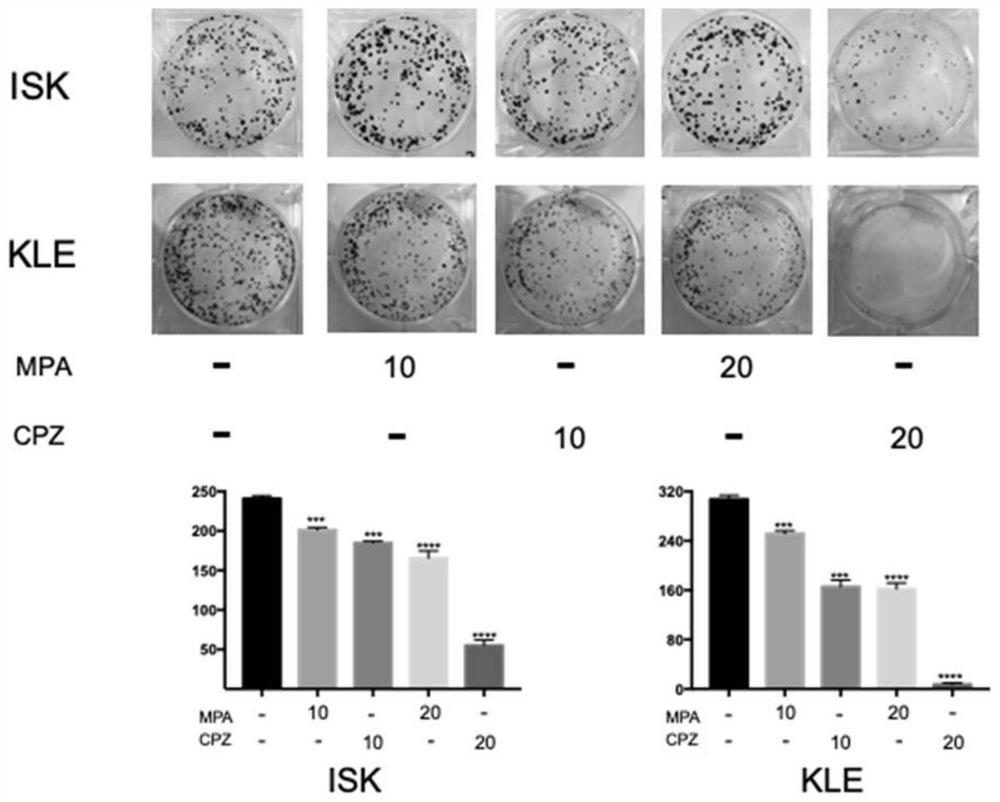
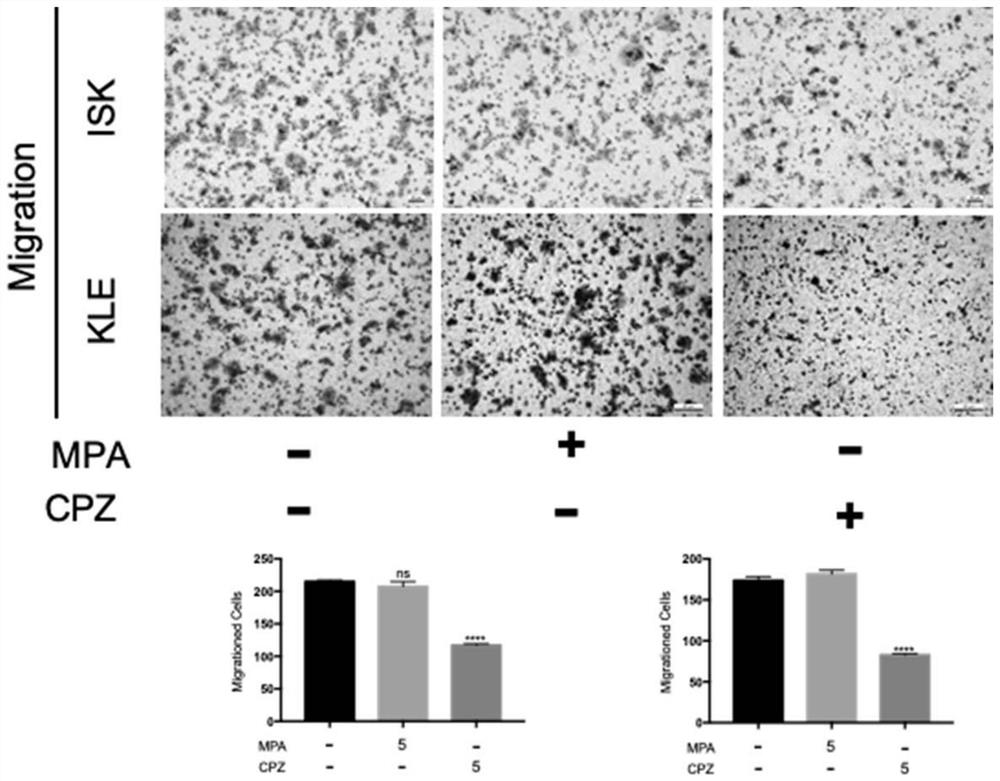
The invention relates to the technical field of medicines, in particular to application of chlorpromazine hydrochloride to treatment of endometrial cancer, and the endometrial cancer comprises type I endometrial cancer and type II endometrial cancer. Experiments find that chlorpromazine hydrochloride has an inhibiting effect on proliferation, clone formation and migration ability of endometrial cancer Ishikawa (ISK cells) and KLE cells, can induce apoptosis, up-regulate cell PRB expression, inhibit cell AKT phosphorylation and inhibit growth of subcutaneous transplantation tumors of mice, and is proved to be effective in mouse experiments. Therefore, the invention provides a new application of the antipsychotic drug chlorpromazine hydrochloride in treatment of endometrial cancer.
Owner:THE INT PEACE MATERNITY & CHILD HEALTH HOSPITAL OF CHINA WELFARE INST +1
Chlorpromazine tablet and preparation method thereof
InactiveCN108451918AHigh dissolution rateReduce typesOrganic active ingredientsNervous disorderDrug release rateFiller Excipient
Owner:HUAYI PHARMA ANHUI CO LTD
A kind of preparation method of chlorpromazine hydrochloride multicapsule sustained-release granule
ActiveCN105125523BRelease stabilityUniform sizeOrganic active ingredientsNervous disorderPrillFreeze-drying
Owner:嘉兴天源药业有限公司
Method for determining residual sedative type veterinary medicaments in mutton
InactiveCN103954721BMeet Residue Analysis RequirementsHigh recovery rateComponent separationPerphenazinePromethazine
The invention relates to a method for determining residual medicaments in mutton, and in particular relates to a method for determining multiple sedative type medicaments in mutton at the same time. The residual sedative type veterinary medicaments refer to zolpidem, haloperidol, chlordiazepoxide, promethazine, nitrazepam, chlorpromazine hydrochloride, perphenazine, fluphenazine hydrochloride, clonazepam, xylazine hydrochloride, propionylpromazine, carazolol, acepromazine, droperidol and azaperone. The method has the advantage that the residual amounts of 15 types of sedative type medicaments in mutton are determined through high-resolution liquid chromatography-tandem mass spectrometry. The method is high in sensitivity and high in recovery rate, and can meet the detection requirements on veterinary medicaments.
Owner:GANSU AGRI UNIV
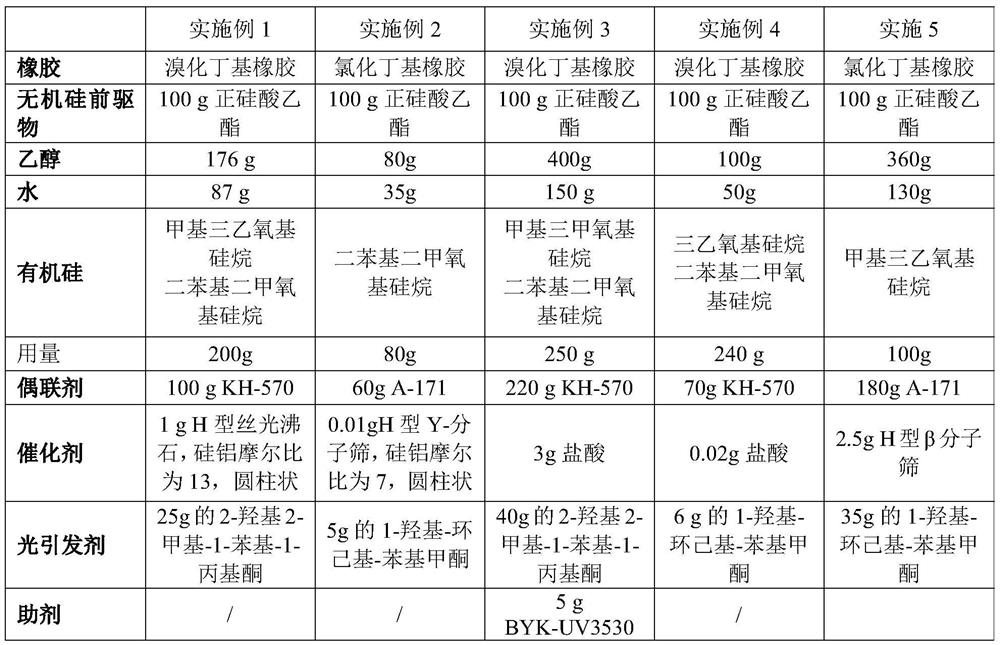
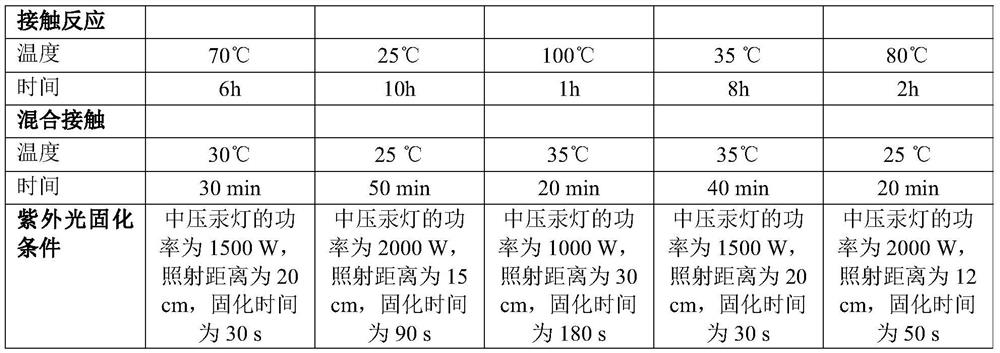
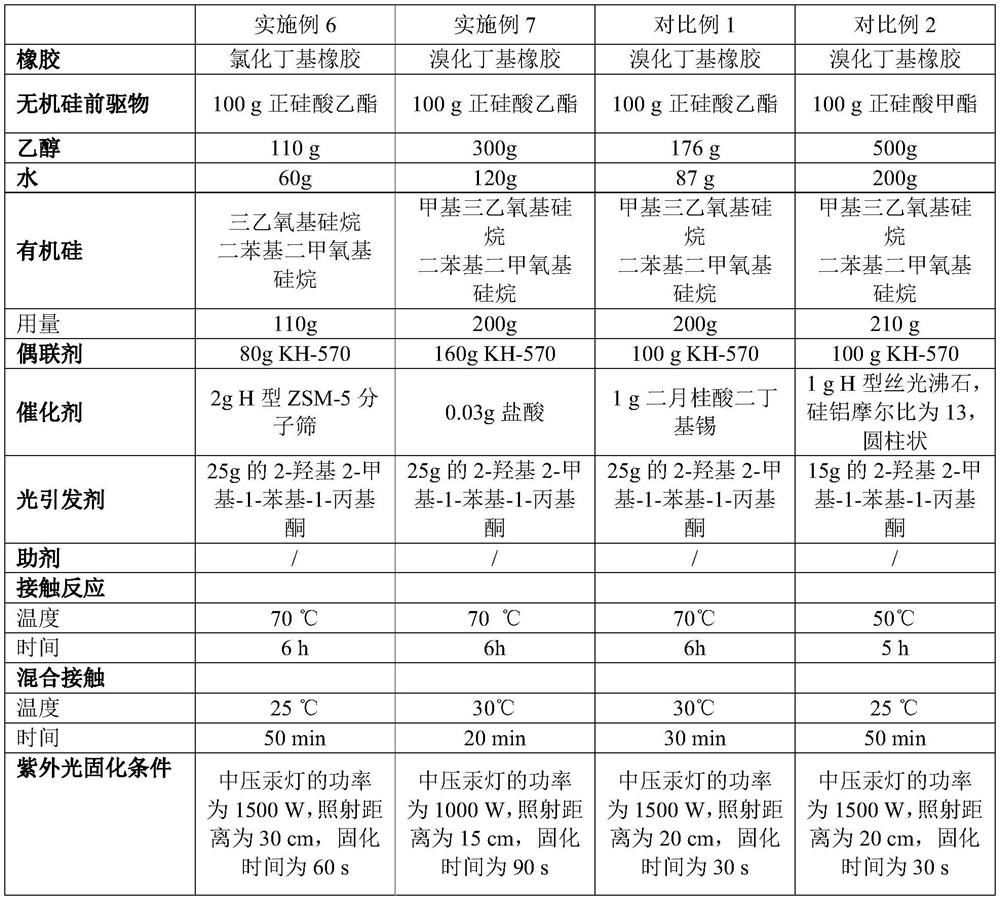
Coating liquid composition, coating liquid, film-coated rubber plug and preparation method of film-coated rubber plug
PendingCN114644882ASimple preparation processQuality is not affectedPharmaceutical containersMedical packagingPtru catalystUltraviolet lights
The invention relates to the technical field of medicinal rubber plug production, and discloses a coating liquid composition, a coating liquid, a film-coated rubber plug and a preparation method of the film-coated rubber plug, the composition contains an inorganic silicon precursor, ethanol, water, organic silicon and a coupling agent; wherein the preparation method of the silicon film is an ultraviolet light curing method; relative to 100 parts by weight of the inorganic silicon precursor, the content of the ethanol is 80 to 400 parts by weight, the content of the water is 35 to 150 parts by weight, the content of the organic silicon is 80 to 250 parts by weight, the content of the coupling agent is 60 to 220 parts by weight, and the content of the catalyst is 0.01 to 3 parts by weight. The film-coated rubber plug provided by the invention is low in surface friction coefficient, and the coating on the surface of the film-coated rubber plug does not fall off, so that the film-coated rubber plug has better resistance to common drugs such as chlorpromazine hydrochloride.
Owner:CHINA PETROLEUM & CHEM CORP +1
A method for staining the anterior lens capsule in overmature cataract surgery
ActiveCN109481141BNo drug chemical toxicityNo complicationsEye surgerySurgical veterinaryTreatment and control groupsBiocompatibility
The invention belongs to the field of medical technology, and discloses a method for staining the anterior lens capsule in overmature cataract surgery. Take 240 clean-grade male Wistar rats with a body weight of 200-250 g; the animals are randomly divided into a normal control group and an experimental group; The experimental group was dilated with compound tropicamide eye drops three times before operation, anesthetized by intramuscular injection of a mixture of ketamine hydrochloride and chlorpromazine hydrochloride, then made an upper limbal incision, injected methylene blue injection into the anterior chamber, and stayed in the anterior chamber Afterwards, fully flush with compound sodium chloride injection; inject appropriate amount of viscoelastic agent, continuously circular capsulorhexis, observe tissue contrast; continue capsulorhexis after trimming with capsular shears. The present invention does not require additional preparation, can be taken at any time, and has good biocompatibility; it has no drug chemical toxicity to intraocular tissues, such as corneal endothelial cells and visual cells; residual traces can be discharged through normal intraocular absorption or aqueous humor circulation; no Obvious complications.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Simultaneous detection of 7 kinds of sleep chemicals detection method
The invention discloses a supplemental detection method of seven chemical medicines including carbamazepine, chlorpromazine hydrochloride, olanzapine, doxepin hydrochloride, quetiapine fumarate, oxcarbazepine and sulpiride which are illegally added into health-care food or Chinese patent medicine for improving sleep. After a sample is subjected to ultrasonic extraction with methyl alcohol, chromatogram column separation is conducted, a mobile phase is eluted, and DAN detector is used for detection. By means of a built detection method, methodological verification is conducted, and parameters of results are shown in the description. It is verified that the method is quick, high in specificity and suitable for detection of chemical medicine added to the health-care food or Chinese patent medicine for improving sleep.
Owner:SHANXI PROVINCE FOOD & DRUG INSPECTION INST
Pharmaceutical composition for treating dysmenorrhoea
InactiveCN106729602AMeeting urgent needsLittle side effectsHydrolysed protein ingredientsSexual disorderPhysiologyGlucocorticoid
The invention discloses a pharmaceutical composition for treating dysmenorrhoea. The pharmaceutical composition comprises the following raw materials in parts by weight: 2-5 parts of paracetamol, 2-5 parts of sulpiride, 0.1-2 parts of atropine, 0.8-5 parts of EGCG, 1-5 parts of citric acid, 0.1-1 part of chlorine nitrogen, 5-10 parts of olive oil, 2-5 parts of chlorpromazine hydrochloride, 0.2-1 part of vitamin p, 1-5 parts of earthworm protein powder, 5-9 parts of donkey-hide gelatin, 1-5 parts of diclofenac sodium, 2-8 parts of vitamin E, 2-5 parts of glucocorticoid medicine, and 80-150 parts of physiological saline. The pharmaceutical composition for treating dysmenorrhoea develops a synergistic effect by means of combination of the raw materials, is capable of effectively treating symptoms such as stomachache, soreness and weakness of waist, and cool hands and feet during menstruation, and is simple to prepare and low in production cost. The medicine is capable of thoroughly treating both manifestation and root cause of dysmenorrhoea without causing drug dependence of a patient, and is low in side effects without complication.
Owner:郑州莉迪亚医药科技有限公司
Method for processing medicament for relieving animal transportation stress response
InactiveCN102949396AAvoid damageAvoid economic lossOrganic active ingredientsMetabolism disorderWater buffaloPhysiology
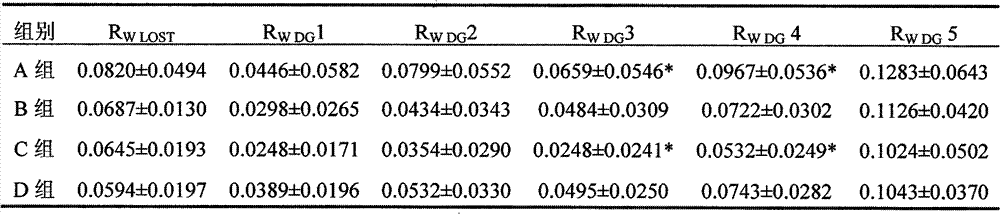
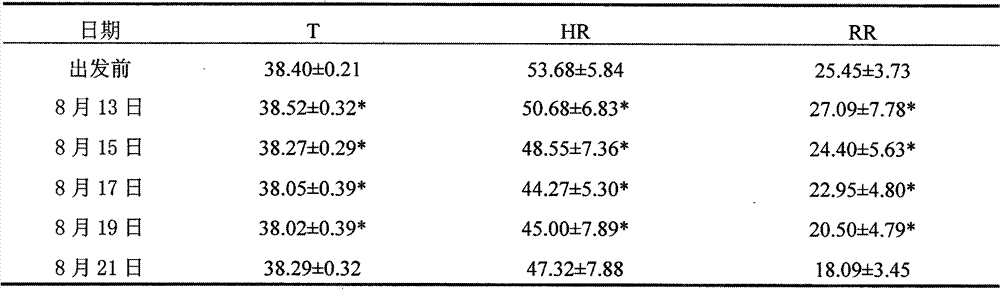
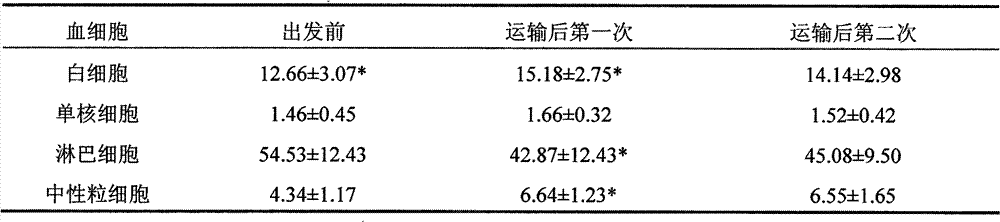
The invention belongs to the technical field of transporting commercial animals, and provides a method for processing a mixed medicament for relieving long-distance transportation stress response of commercial animal, especially water buffalo ox. The method is characterized by comprising the following steps by weight: applying 1mg / kg chlorpromazine hydrochloride plus 1g / kg white spirit with alcoholic strength of 50 degrees (V / V) or 1mg / kg chlorpromazine hydrochloride plus 2g / kg white spirit with alcoholic strength of 50 degrees (V / V) or 1mg / kg chlorpromazine hydrochloride plus 3g / kg white spirit with alcoholic strength of 50 degrees (V / V) to transported animal in transportation of animals before second hour. After being determined by transportation stress indexes such as growth indexes of weightlessness and average daily gain, body mass indexes of heart rate and respiratory rate and body temperature, immunity index WBC (White Blood Count) and behavior index of ingestion times and the like, the weight recovery period after long-distance transportation stress is determined to be at least 9d, and adverse reaction drugs measures for relieving the transportation stress can be provided. The method can be used for avoiding damage of long-distance transportation stress to the health and welfare.
Owner:HUAZHONG AGRI UNIV
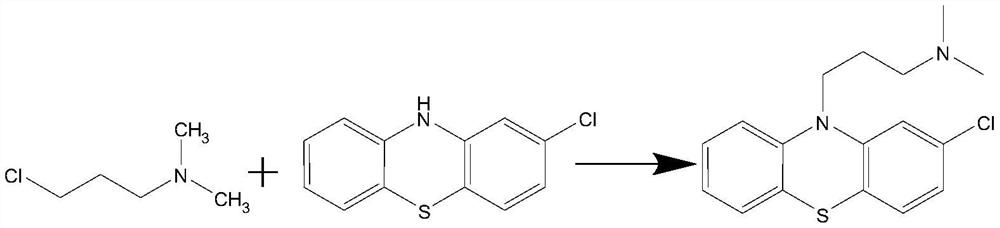
Preparation method of chlorpromazine hydrochloride
The invention relates to a preparation method of chlorpromazine hydrochloride, and belongs to the technical field of medicine preparation. According to the method, 2-chlorophenothiazine and N, N-dimethyl-3-chloropropylamine are used as raw materials, an N-methyl pyrrolidone organic solvent is adopted, chlorpromazine is generated under the action of a 4-dimethylaminopyridine catalyst, the obtained chlorpromazine and hydrogen chloride gas are salified, and chlorpromazine hydrochloride is obtained. According to the method, a toxic toluene reagent is not needed, and an N-methyl pyrrolidone organic solvent is adopted, so that the method is more environment-friendly, and is more beneficial to recovery of chlorpromazine, and the yield and purity of chlorpromazine are improved. A large amount of sodium hydroxide does not need to be added, sodium hydroxide and tetrabutylammonium bromide in an original process can be replaced by selecting 4-dimethylaminopyridine, and the yield and purity of chlorpromazine hydrochloride are remarkably improved. By controlling the conditions of the salt forming reaction of hydrochloric acid, the crystallization and purification of chlorpromazine hydrochloride are facilitated, and high-purity chlorpromazine hydrochloride can be obtained.
Owner:常州康普药业有限公司 +1
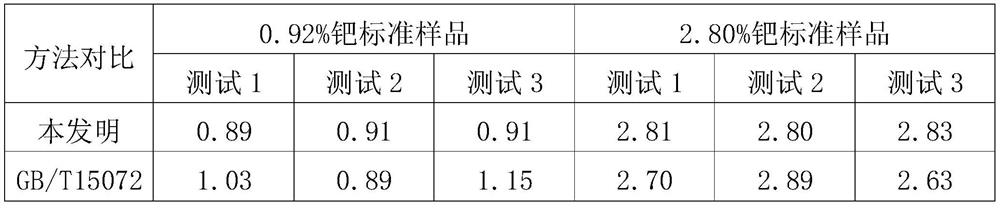
Analysis method for determining content of palladium in waste residues
PendingCN112730289AImprove detection accuracyReduce testing costsPreparing sample for investigationColor/spectral properties measurementsDistilled waterVolumetric flask
The present invention discloses an analysis method for determining content of palladium in waste residues. The analysis method comprises the following steps: calcining a waste residue sample at high temperature, cooling to room temperature, adding a mixed solution of hydrochloric acid and nitric acid, heating at low temperature until the mixed solution is slightly boiled, sequentially adding sodium chloride and 10% hydrochloric acid, completely dissolving, transferring into a 100ml volumetric flask, and diluting with distilled water to a scale to obtain a pretreated sample solution; adding 1ml of the pretreated sample solution into a 60ml separating funnel, adding 20ml of water, adjusting the pH value to be 2 by using a 5% hydrochloric acid solution and a 5% sodium hydroxide solution, adding 2ml of a 1% chlorpromazine hydrochloride solution, uniformly shaking and standing for 10min to generate a red complex, adding 10ml of trichloromethane, carrying out oscillation extraction for 1min, taking a lower trichloromethane solution after layering, and taking reagent blank as a reference; and measuring the absorbance of the solution at 488 nm by using a 1 cm cuvette, and calculating the palladium content according to the absorbance and the standard regression curve. The method is low in detection cost and can be suitable for detecting the content of 0.1%-5% palladium.
Owner:CHONGQING THRIVE CHEM
A kind of preparation method of chlorpromazine hydrochloride
The invention relates to a preparation method of chlorpromazine hydrochloride, which belongs to the technical field of medicine preparation. The present invention takes 2-chlorophenothiazine and N, N-dimethyl-3-chloropropylamine as raw materials, adopts N-methylpyrrolidone organic solvent, reacts under the effect of 4-dimethylaminopyridine catalyst, and generates chlorine Promethazine, the gained chlorpromazine and hydrogen chloride gas form a salt, namely get chlorpromazine hydrochloride. The present invention does not need to use toluene toxic reagent, and adopts N-methylpyrrolidone organic solvent, which is not only more environmentally friendly, but also more conducive to the recovery of chlorpromazine, and improves the yield and purity of chlorpromazine. And without adding a large amount of sodium hydroxide, by selecting 4-dimethylaminopyridine, it can replace sodium hydroxide and tetrabutylammonium bromide in the original process, and significantly improve the yield and purity of chlorpromazine hydrochloride. Controlling the conditions of the hydrochloric acid salt-forming reaction is more favorable for the crystallization and purification of chlorpromazine hydrochloride, and higher purity chlorpromazine hydrochloride can be obtained.
Owner:常州康普药业有限公司 +1
Method for increasing yield of dibasic acid fermentation
InactiveCN109694888AImprove conversion rateAttenuates alpha-oxidative decarboxylationMicroorganism based processesFermentationAlkaneCarbon number
The invention relates to a method for increasing a yield of dibasic acid fermentation, which uses C10-C18 n-alkane as a raw material and employs a microbial fermentation method. The method is characterized by the addition of an alpha-oxidative decarboxylation inhibitor and / or a beta-oxidation inhibitor during fermentation production is used to attenuate the alpha-oxidative decarboxylation and beta-oxidicability of the strain, the alpha-oxidative decarboxylation inhibitor is one or a mixture of chlorpromazine hydrochloride, sodium phenobarbital, acrylic acid or polyacrylic acid, and the beta-oxidation inhibitor is one or a mixture of thioglycolic acid, sodium thioglycolate, ranolazine, ranolazine dihydrochloride or rice koji. The method has the beneficial effects that in the process of producing dibasic acid with the corresponding carbon number by n-alkane fermentation, the inhibitor is used to weaken the alpha-oxidative decarboxylation and beta-oxidation ability of the strain, and reduces the unit consumption of the alkane, and the alkane fermentation conversion rate is greatly improved.
Owner:陈凯南
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com