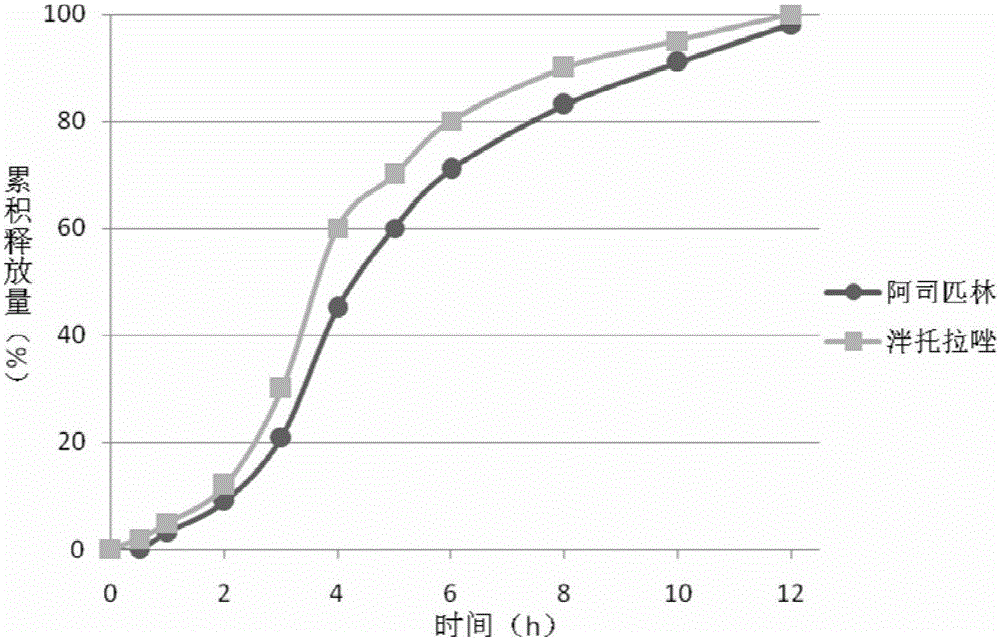
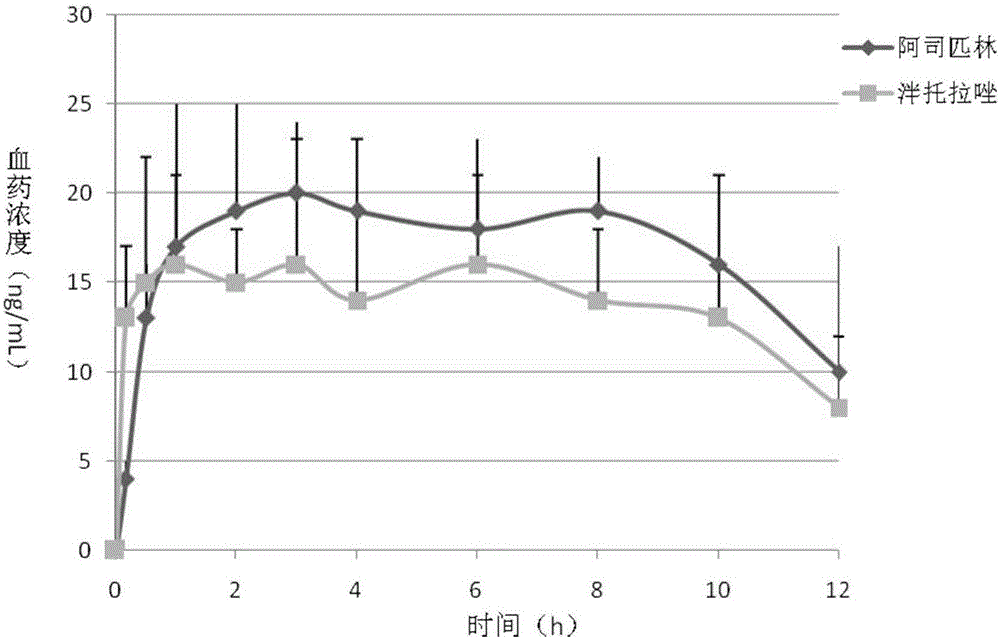
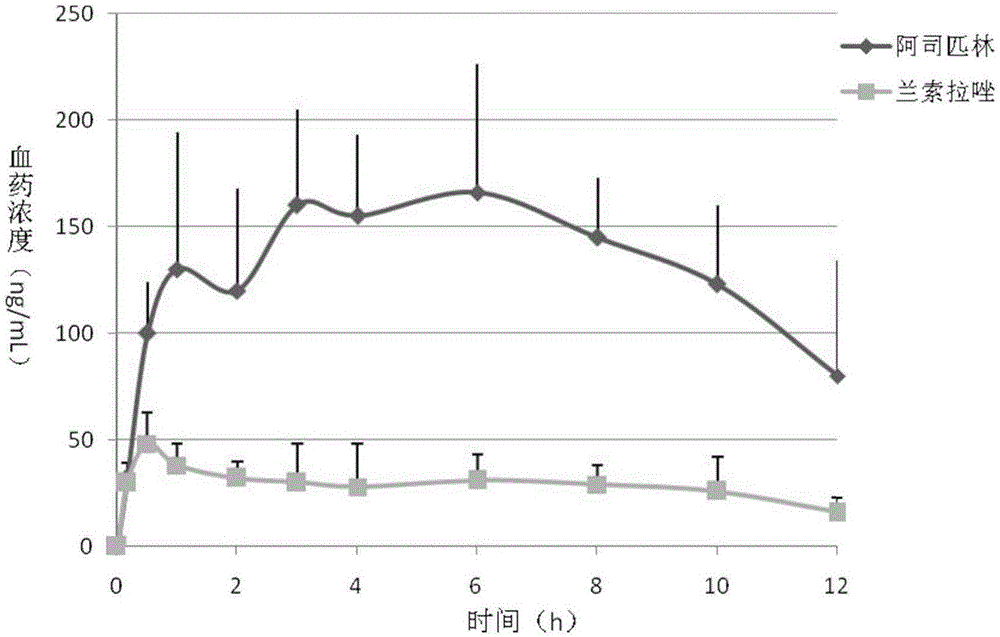
Compound capsule containing aspirin and proton pump inhibitor
A technology of proton pump inhibitors and aspirin, which is applied in the field of compound capsules containing aspirin and proton pump inhibitors, can solve the problems of instability of proton pump inhibitors, loss of protection of proton pump inhibitors in gastrointestinal mucosa, and short half-life, etc. Achieve the effect of being conducive to industrialized large-scale production, convenient for quality control, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] The preparation method of the compound capsule containing aspirin and proton pump inhibitor of the present invention comprises the following steps:
[0069] The first part: Mix aspirin, stabilizer, porogen and skeleton material according to the prescription ratio, add an appropriate amount of adhesive to prepare a soft material, extrude through an extruder, and place the strip in a spheronizer to spheroidize. After the spheronized pellets are heat-treated and dried, they are sieved for later use. Prepare HPMC solution and add anti-adhesive agent and opacifier according to the prescription ratio to prepare a coating solution for the isolation layer, and place the prepared aspirin pill core in a fluidized bed to coat the isolation coating. Prepare the enteric coating solution according to the prescription ratio and wrap it on the above-mentioned isolation coat.
[0070] The second part: mix the proton pump inhibitor, stabilizer and filler of the prescribed amount evenly,...
Embodiment 1
[0075] first part
[0076]
[0077]
[0078] the second part
[0079]
[0080] the third part
[0081]
[0082] Preparation:
[0083] ① Mix aspirin, tartaric acid, lactose, stearic acid and ethyl cellulose evenly according to the prescription ratio, add 10% starch slurry to prepare a soft material, extrude through an extruder, and place the strip in a spheronizer to spheroidize. The pellets after spheronization are dried in an oven and sieved for later use. Prepare HPMC solution (100mL) and add talcum powder and titanium dioxide according to the prescription ratio to prepare the isolation layer coating liquid, and place the prepared aspirin-containing pill core in a fluidized bed to coat the isolation coat. Prepare the enteric coating solution (50 mL) according to the prescription ratio and wrap it on the above-mentioned isolation coat.
[0084] 2. Pantoprazole, sodium bicarbonate, and sucrose are mixed homogeneously with prescription quantity, prepare pantopra...
Embodiment 2
[0088] first part
[0089]
[0090]
[0091] the second part
[0092]
[0093] the third part
[0094]
[0095]
[0096] Preparation method ①, ②, ③ are the same as in Example 1.
[0097] Preparation method ④: Weigh about 700 mg of the first part, about 40 mg of the second part, and about 150 mg of the third part, mix them evenly, and fill them in gelatin capsule shells to obtain compound capsules containing aspirin and esomeprazole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com