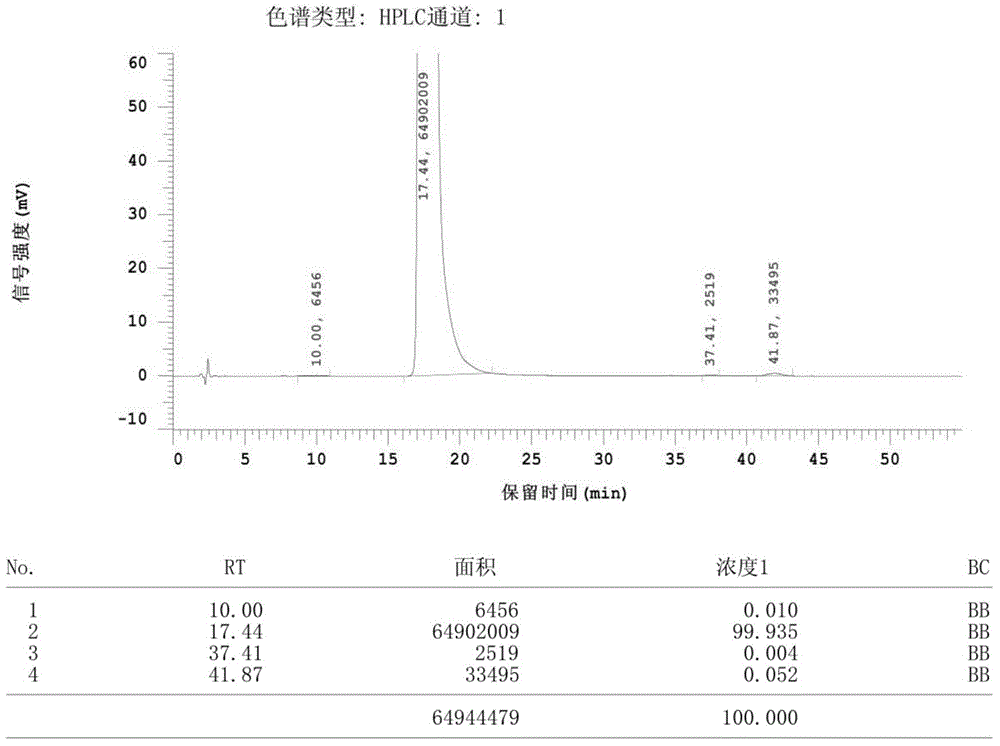
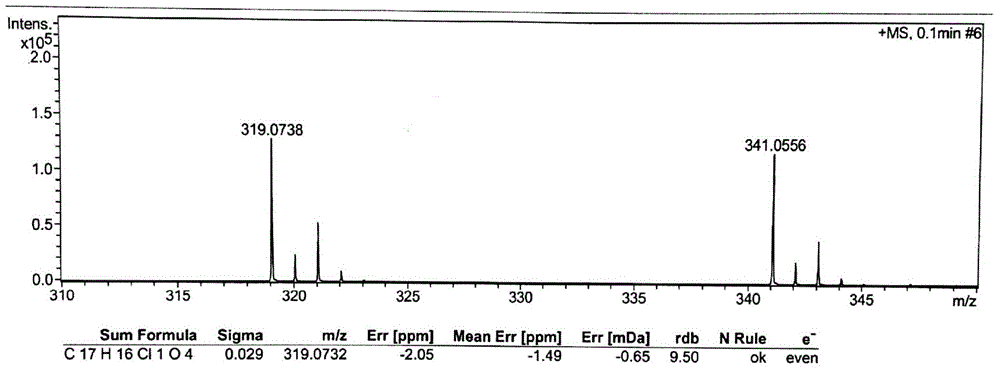
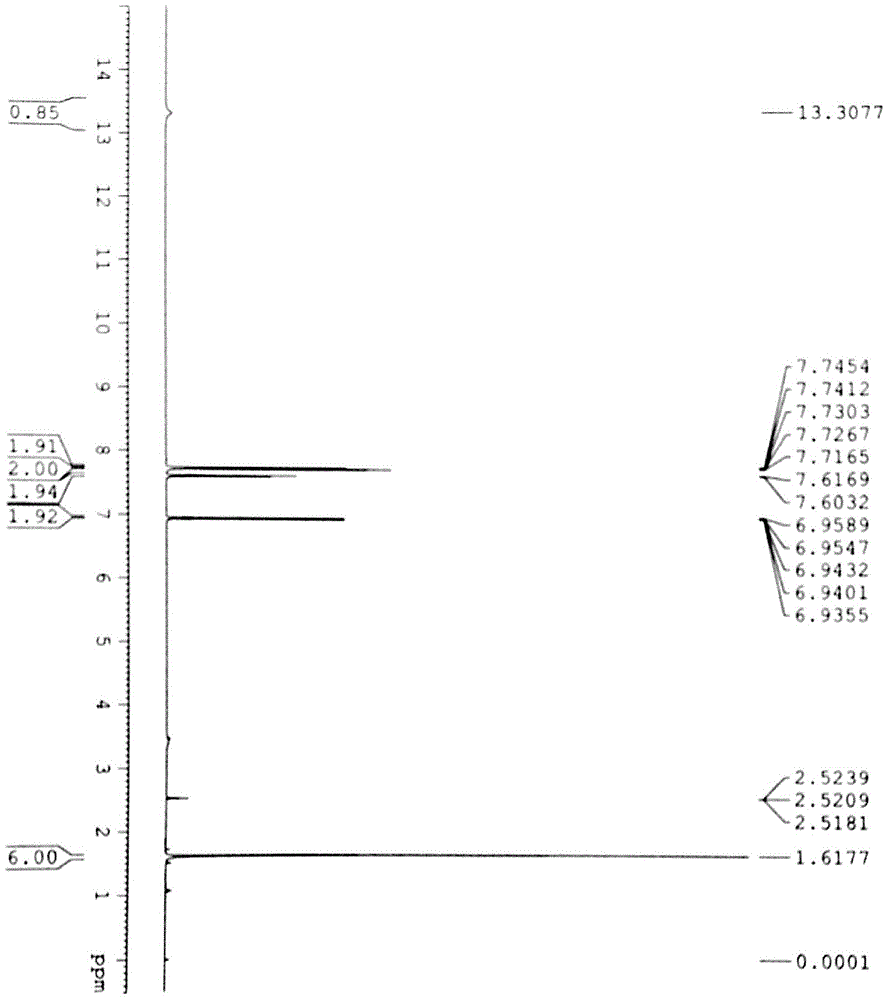
Preparation method for high-purity fenofibric acid crude drugs
A fenofibric acid, high-purity technology, applied in the synthesis method and refining field of fenofibric acid, can solve the problems of low yield, low product purity, and many times of refining, and achieve the effect of mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of 4'-chloro-4-methoxybenzophenone (II)
[0032] Add 13.8g of anisole, 70ml of dichloromethane, and 25.5g of anhydrous aluminum trichloride into the reaction flask, stir at -5~0°C, add 29.0g of p-chlorobenzoyl chloride dropwise (with an equal volume of dichloro Diluted with methane), after dropping, the temperature was raised to room temperature for 2 hours, and the reaction was stopped. The reaction solution was added into ice water, stirred evenly, extracted with 50ml of dichloromethane to obtain a dichloromethane layer, evaporated to dryness to obtain a white solid, dried to constant weight, and weighed to obtain 29.3g, yield 93.2%.
[0033] Preparation of 4'-chloro-4-hydroxybenzophenone (I)
[0034] Add 29.0g of (II), 32.9g of anhydrous aluminum trichloride, and 90ml of chlorobenzene to the reaction flask in sequence. Cool at room temperature, concentrate to dryness at 70~90℃, -0.05~-0.085Mpa, let cool, add dichloromethane to dissolve, add ice water to...
Embodiment 2
[0055] Preparation of 4'-chloro-4-methoxybenzophenone (II)
[0056] Add 10.6g of anisole, 19.6g of anhydrous aluminum trichloride, and 60ml of dichloromethane into the reaction flask, stir at -10 to 0°C, add 22.3g of p-chlorobenzoyl chloride dropwise (with an equal volume of dichloromethane Diluted with methane), after dropping, the temperature was raised to room temperature and reacted for 3 hours, and the reaction was stopped. The reaction solution was added into ice water, stirred evenly, extracted with 50 ml of dichloromethane to obtain an organic layer, evaporated to dryness to obtain a white solid, dried to constant weight, and weighed to obtain 22.9 g, yield 94.6%.
[0057] Preparation of 4'-chloro-4-hydroxybenzophenone (I)
[0058] Add 12.6g of (II), 16.2g of anhydrous aluminum trichloride, and 40ml of chlorobenzene to the reaction bottle in sequence. After the addition, the temperature rises to 100°C. After heating and stirring for 2 hours, after the reaction is comp...
Embodiment 3
[0070] Preparation of 4'-chloro-4-methoxybenzophenone (II)
[0071] Add 13.2 g of anisole, 35 ml of anhydrous boron trifluoride ether solution, and 80 ml of dichloromethane into the reaction flask, stir at -10 to 0°C, add 25.6 g of p-chlorobenzoyl chloride dropwise (with an equal volume of dichloromethane Chloromethane dilution), dropwise, warming up to room temperature to react for 4 hours, stop the reaction. The reaction solution was added into ice water, stirred evenly, extracted with 60 ml of ethylene glycol monomethyl ether to obtain an organic layer, evaporated to dryness to obtain a white solid, dried to constant weight, and weighed to obtain 26.0 g, yield 85.6%.
[0072] Preparation of 4'-chloro-4-hydroxybenzophenone (I)
[0073] Add 15.3g of (II), 120ml of boron tribromide in methylene chloride (containing 50g of boron tribromide), and 50ml of chlorobenzene to the reaction flask in sequence. After the detection reaction is complete, place the reaction solution at ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com