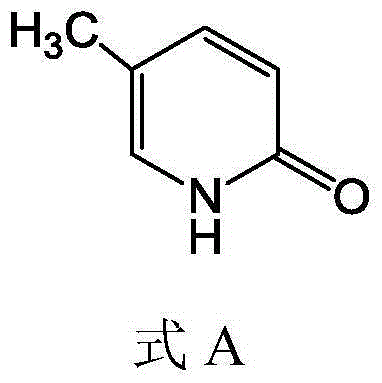
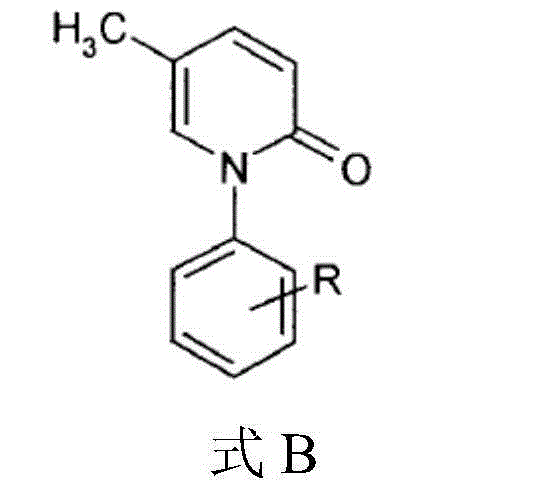
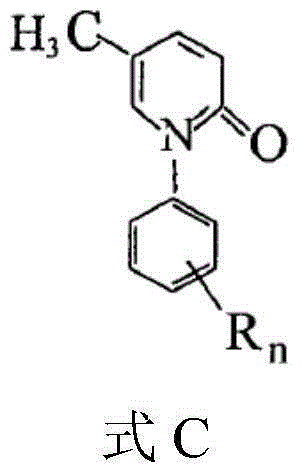
5-methyl-2(1H)pyridone derivatives, preparation method and applications thereof
A technology of pyridone and derivatives, applied in the field of 5-methyl-2 pyridone derivatives and preparation thereof, can solve the problem of the preparation of 5-methyl-2(1H) pyridone derivatives that have not been seen or seen methods and uses to achieve the effect of simple steps, low cost and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081]
[0082] Among them, "rf" is the abbreviation of reflux, and its Chinese meaning is "reflux".
[0083] In a 25ml reaction bottle, first add 3.4ml from 17mlH 2 O and the solution (50%, volume fraction) that 17ml concentrated sulfuric acid forms, then add 1g (0.01mol) 2-amino-5-picoline, cool to below 10 ℃ with ice-salt bath, after stirring several minutes, reaction solution Turned milky white. Then slowly drop by (1.72gNaNO 2 with 3mLH 2 O) the mixed solution, during dropwise addition, produces irritating gas, after dropwise addition, the reaction solution turns into a light yellow solution, and TCL (thin layer chromatography) monitors until the reaction is complete (about 40min). Then add 8mLH 2 O, reflux stirring reaction 15min, cooling, add anhydrous Na under stirring 2 CO 3 , make the reaction solution neutral (produce yellow-brown solid), filter, the gained filtrate is spin-dried, then dissolve and filter with absolute ethanol, and the gained filtrate is sp...
Embodiment 2
[0093]
[0094] According to the method described in Example 1, replacing "p-methylacetophenone" with "p-chloroacetophenone", the compound shown in formula Ib was obtained.
[0095] Compound represented by formula Ib: yellow solid (yellow solid), melting point (mp) is 66-67°C;
[0096] 1 HNMR (400MHz, DMSO-d6) δ: 8.02 (d, J = 8.8Hz, 4H, ArH), 7.62 (d, J = 8.4Hz, 4H, ArH), 7.51 (d, J = 8.4Hz, 2H, ArH ), 7.37~7.40(m, 2H, CH), 7.30(d, J=8.4Hz, 2H, ArH), 6.43(d, J=10.0Hz, 1H, CH), 3.97~4.03(m, 2H, CH ), 3.55 (d, J=6.8Hz, 4H, CH 2 ),2.06(s,6H,CH 3 );
[0097] 13 CNMR (100MHz, DMSO-d6) 197.40, 160.34, 143.95, 139.04, 136.02, 135.26, 129.84, 129.76, 126.31, 120.11, 113.91, 44.21, 39.53, 35.53, 16.23; IR (KBr, n, cm -1 ):3429,1673,1590,1400,1262,1091,804,590;
[0098] HRMS(ESI)calcdforC 29 h 23 Cl 2 NO 3 [M+Na] + 503.1055found526.0963.
Embodiment 3
[0100]
[0101] According to the method described in Example 1, replacing "p-methylacetophenone" with "p-fluoroacetophenone", the compound represented by formula Ic was obtained.
[0102] Compound represented by formula Ic: gray solid (gray solid), melting point (mp) is 60-62 ° C;
[0103] 1 HNMR (400MHz, DMSO-d6) δ: 8.06(dd, J1=6.0Hz, J2=8.4Hz, 4H, ArH), 7.48(d, J=8.4Hz, 2H, CH), 7.26~7.35(m, 8H ,ArH),6.40(d,J=10.0Hz,1H,CH),3.97~4.04(m,1H,CH),3.52(d,J=6.8Hz,4H,CH 2 ),2.02(s,6H,CH 3 );
[0104] 13 CNMR(100MHz,DMSO-d6)196.93,166.24,163.74,160.35,144.06,142.81,139.02,136.01,133.37,130.95,128.14,126.30,120.11,115.73,113.91,44.17,39.11,35.87,16.21;IR(KBr, n,cm -1 ):2925,1675,1596,1506,1410,1364,1277,1230,1156,990,832,586;
[0105] HRMS(ESI)calcdforC 29 h 23 f 2 NO 3 [M+Na] + 471.1646found494.1549.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com