Supported cyclometalated iridium catalyst, preparation method thereof and application of supported cyclometalated iridium catalyst in dehydrogenation reaction of dihydroindolines compounds
A technology for catalyzing indoline and metal iridium, which is applied in the field of catalysis, can solve the problems of restricting the large-scale application of catalysts, difficulty in recycling, and difficulty in effective separation, and achieves the effects of short preparation cycle, high catalytic activity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
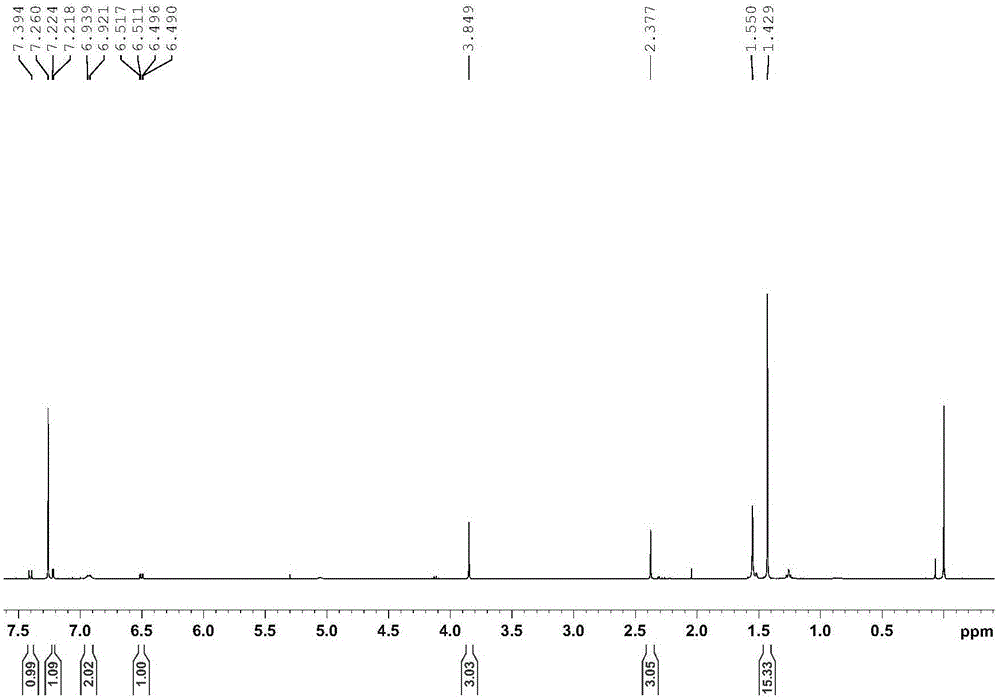
Embodiment 1
[0031] 1. Synthesis of 4-methoxy-N-[1-(4-hydroxyphenyl)methylene]-aniline
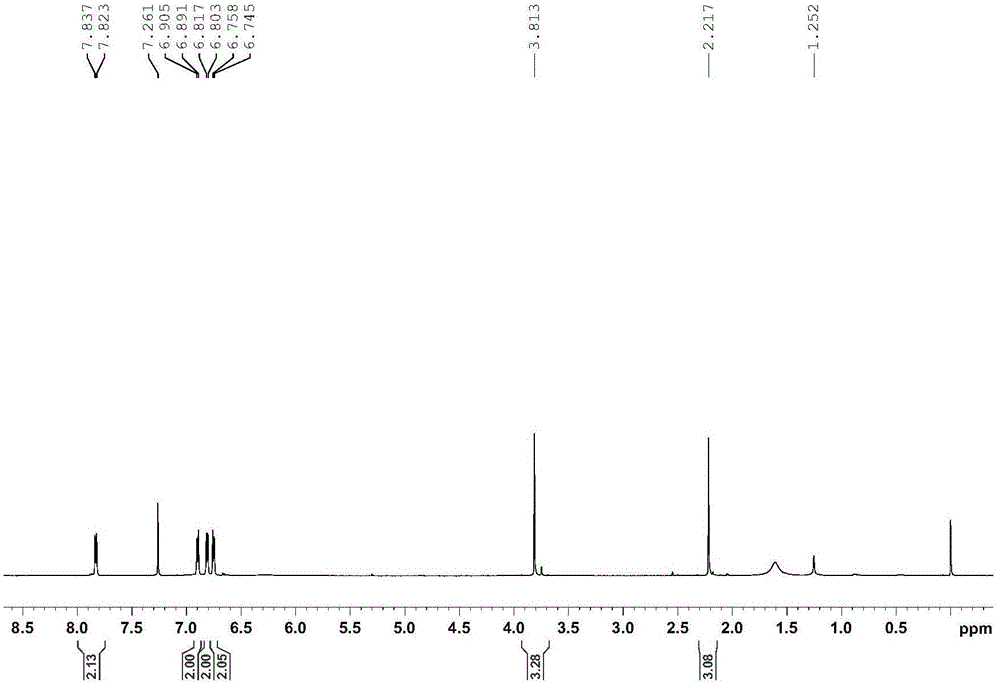
[0032] With 2.72g p-hydroxyacetophenone, 2.95g p-methoxyaniline, 8.4g sodium bicarbonate, 12g activated Molecular sieves and 20mL of toluene were added to the flask in sequence, and heated to reflux for 72 hours under an argon atmosphere at a stirring speed of 1000r / min. Spin to dry, then recrystallize and purify with petroleum ether and ethyl acetate, then remove the mother liquor, and dry in vacuum at 40°C for 12 hours to obtain a light yellow solid 4-methoxy-N-[1-(4-hydroxyphenyl) Methyl]-aniline with a yield of 81.2%.
[0033] The Bruker600MHz superconducting Fourier digital nuclear magnetic resonance spectrometer of Bruker Company is used to measure the proton-nuclear magnetic spectrum of the light yellow solid obtained above, and its proton-nuclear magnetic spectrum is as follows figure 1 As shown, the NMR data are: 1 HNMR (600MHz, CDCl 3 )δ=7.83(d, J=8.4Hz, 2H), 6.90(d, J=8.4Hz, 2H), 6.81(d...
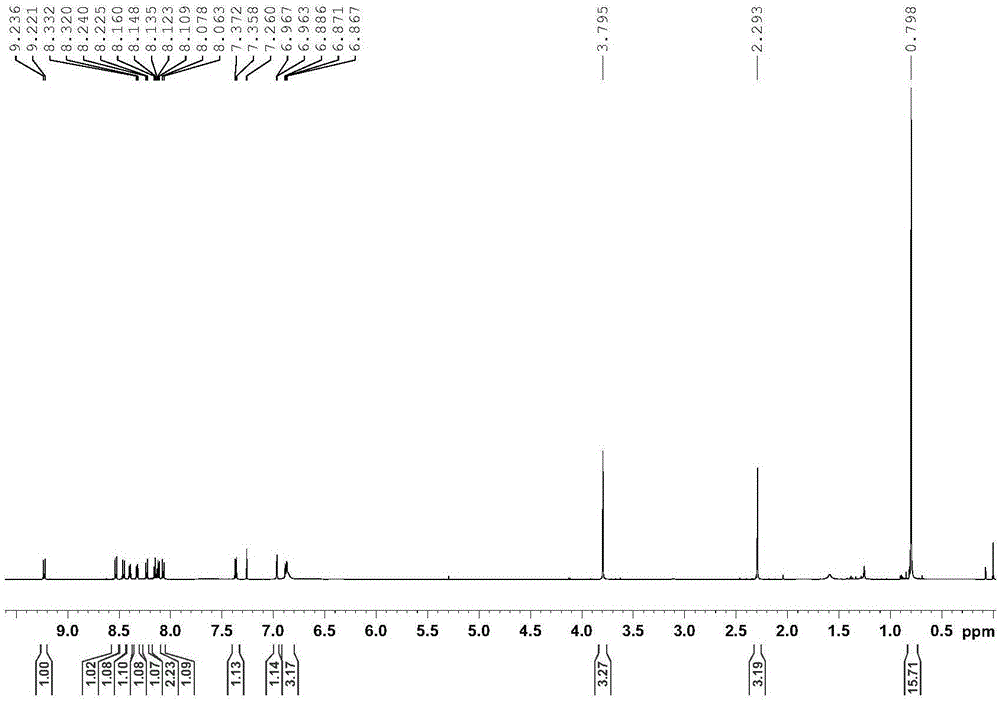
Embodiment 2
[0045] The use of the supported cyclic metal iridium catalyst obtained in Example 1 in catalyzing the dehydrogenation of indoline compounds, the specific method is as follows:
[0046] Add 20 mg of the supported cyclic metal iridium catalyst (the molar weight of the pyrene-labeled cyclic metal iridium complex is 0.005 mmol) into a 30 mL thick-walled pressure-resistant bottle, and then add 0.5 mmol of indoline compounds (see Table 1) and 3mL solvent (V 三氟乙醇 :V 水 =1:2), react at 100°C for 3 hours, cool to room temperature, transfer to a 10mL centrifuge tube with solvent, centrifuge at 10,000r / min for 3min, transfer the centrifugate, then add solvent to the centrifuge tube, shake the centrifuge tube Until the solid-liquid mixture is uniform, centrifuge at 10,000r / min for 3min, combine the centrifuged liquid twice, extract the reaction product with ethyl acetate, and use NMR analysis (internal standard is s-trimethoxybenzene) for dehydrogenation of indoline compounds The convers...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com