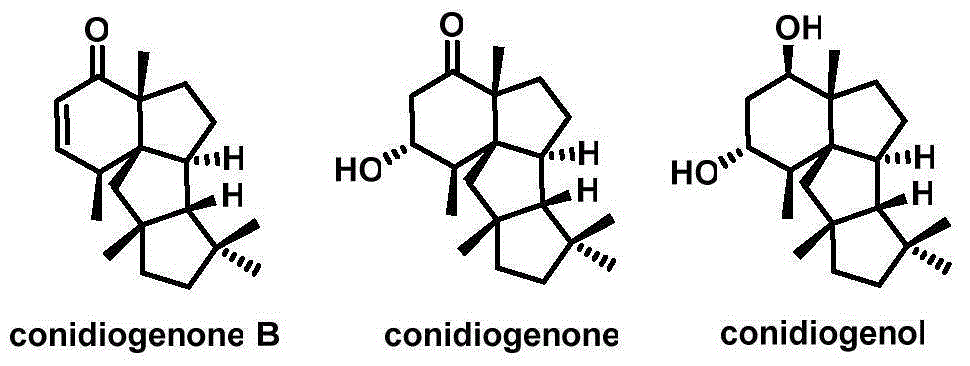
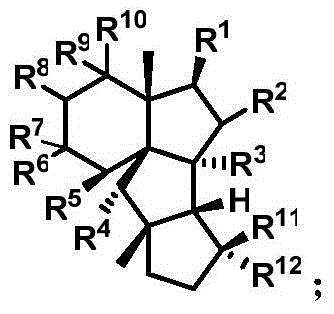
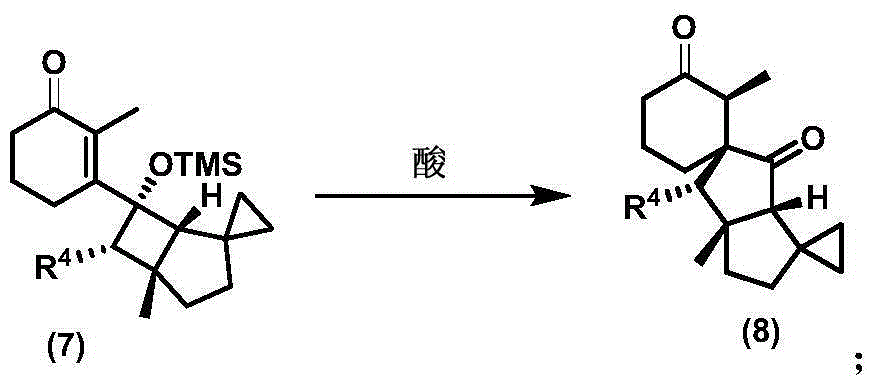
Cyclopiane tetracyclic diterpene analogue, method for synthesizing same and application of cyclopiane tetracyclic diterpene analogue
A technology of a tetracyclic diterpenoid and a synthesis method, applied in the field of medicine and medicine, can solve the problems of less content and no chemical synthesis method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Preparation of compound (2)
[0114] Compound (1) (1.26 g, 10.0 mmol) was dissolved in 17 mL of tetrahydrofuran, under ice methanol bath, cuprous chloride (99 mg, 1.0 mmol) and freshly prepared 1 mol / L 3-methyl-3-butenyl were added successively A solution of magnesium bromide in tetrahydrofuran (14.0 mL, 14.0 mmol) was stirred for 3 hours, water was added to quench the reaction, the reaction solution was extracted with ether, the organic phase was washed with saturated aqueous sodium bicarbonate solution and saturated brine, and the organic phase was then washed with anhydrous sulfuric acid Dry over magnesium, filter, and concentrate the filtrate to obtain crude product. The crude product was dissolved in 20 mL of 2 mol / L aqueous sodium hydroxide solution, stirred at 100 degrees Celsius for 4 hours, extracted with ether, acidified by adding 6 mol / L hydrochloric acid to the aqueous phase until the pH value was 1-2, extracted with dichloromethane, and the organic phase of...
Embodiment 2
[0119] Preparation of compound (3)
[0120] Compound (2) (1.07 g, 0.64 mmol) was dissolved in 10 mL of dichloromethane, oxalyl chloride (0.80 mL, 0.96 mmol) was added, heated to reflux for 2 hours, cooled to room temperature, and the dichloromethane and excess were removed under reduced pressure. Oxalyl chloride. The prepared acid chloride was dissolved in 10 mL of toluene, and a solution of triethylamine (1.32 mL, 0.95 mmol) in 10 mL of toluene was added dropwise under reflux. After the addition was completed, it was cooled to room temperature, ether was added to dilute the system, the reaction was quenched with saturated aqueous ammonium chloride solution, the reaction solution was extracted with ether, the organic phase was washed with saturated aqueous sodium bicarbonate solution and saturated brine, and dried over anhydrous magnesium sulfate, After filtration, the filtrate was concentrated and subjected to column chromatography to obtain a colorless oil (0.58 g, yield 60...
Embodiment 3
[0125] Compound (4) (Substituent R 4 for the preparation of phenylthio)
[0126] Dissolve diisopropylamine (0.22mL, 1.6mmol) in 7mL of tetrahydrofuran, add 2.5mol / L n-butyllithium in n-hexane solution (0.56mL, 1.4mmol) dropwise at minus 78 degrees Celsius, stir halfway under ice-water bath After 1 hour, it was lowered to minus 78 degrees Celsius, and a solution of compound (3) (0.15g, 1.0 mmol) in 1 mL of tetrahydrofuran was added dropwise. After stirring at room temperature for 1 hour, the temperature was reduced to minus 78 degrees Celsius again, and diphenyl disulfide (0.26 g, 1.2 mmol) in 1 mL of hexamethylphosphoric triamide solution, warmed to room temperature and stirred for 10 hours, diluted with diethyl ether, quenched with water, the reaction solution was extracted with diethyl ether, the organic phase was washed with water and saturated brine, and dried over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated and subjected to column chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com