A kind of acrylate monomer with anti-inflammatory effect and preparation method thereof
An acrylate and anti-inflammatory technology, which is applied in anti-inflammatory agents, anti-toxic agents, organic chemistry, etc., can solve the problems of single macromolecular drug structure, unfavorable cell uptake, and lack of functionality, so as to promote the recovery of nerve function and reduce Oxygen free radical generation, effects of inhibiting lipid peroxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
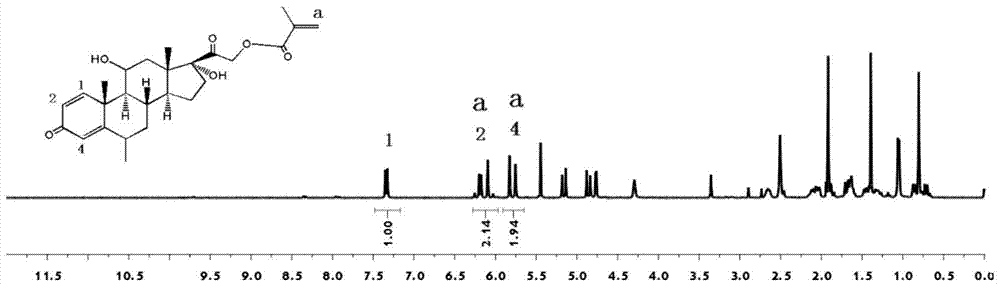
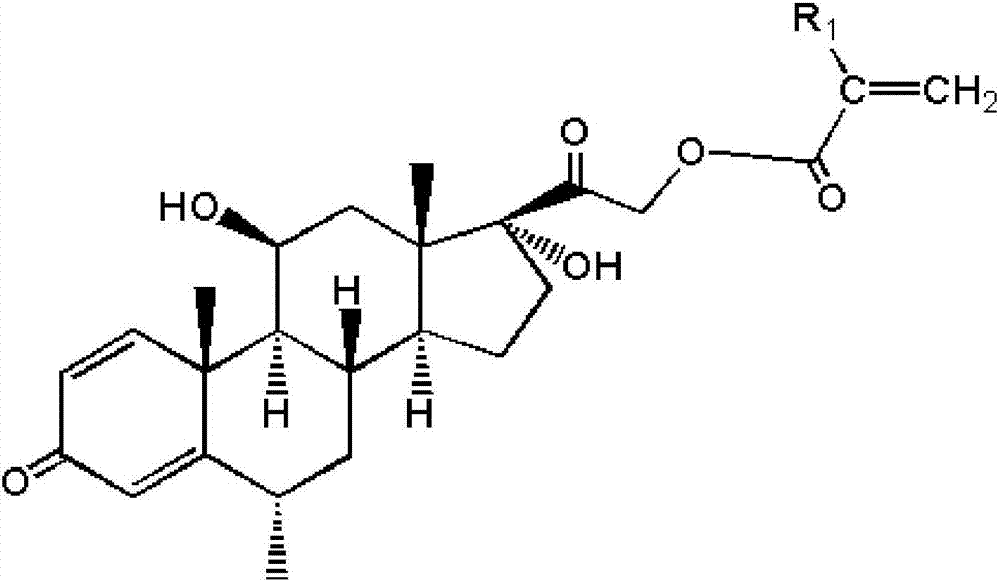
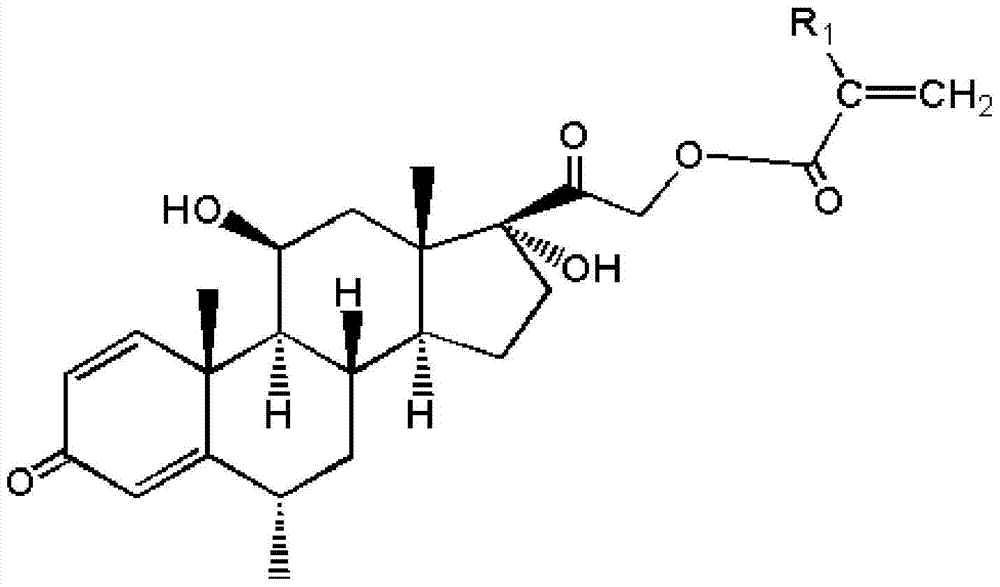
[0038] Dissolve 3mmol methylprednisolone in 10mL N,N-dimethylformamide, then add 5mmol triethylamine, cool down to 0-10°C and keep it at 0-10°C, add 5mL dropwise A solution of acryloyl chloride with a concentration of 0.8 mol / L was reacted in an ice-water bath for 30 minutes after dripping, and then continued to react at room temperature for 2 hours before filtering, adding 20 mL of deionized water to the filtered filtrate, and a solid precipitate was precipitated. After filtering, the filter cake is washed with deionized water until the pH value of the filtrate of the washed filter cake reaches neutral, and the filter cake is vacuum-dried to a constant weight and pulverized to obtain an acrylate functional monomer with a methylprednisolone structure. Wherein, the solution of acryloyl chloride is prepared by dissolving acryloyl chloride in N,N-dimethylformamide.
Embodiment 2
[0040] Dissolve 3mmol of methylprednisolone in 10mL of dimethyl sulfoxide, then add 6mmol of triethylamine, cool down to 0-10°C and keep it at 0-10°C, add 5mL dropwise at a concentration of 1mol / L of methacryloyl chloride solution, after dripping, react in an ice-water bath for 30 minutes, then continue to react at room temperature for 2 hours and then filter, add 20 mL of deionized water to the filtered filtrate, precipitate a solid precipitate, filter, filter The cake is washed with deionized water until the pH value of the filtrate of the washed filter cake reaches neutrality, and the filter cake is vacuum-dried to constant weight and pulverized to obtain an acrylate functional monomer with a methylprednisolone structure. Wherein, the solution of methacryloyl chloride is prepared by dissolving methacryloyl chloride in dimethyl sulfoxide.
Embodiment 3
[0042] Dissolve 3mmol of methylprednisolone in 10mL of dimethyl sulfoxide, then add 6mmol of anhydrous sodium carbonate, cool down to 0-10°C and keep it at 0-10°C, add 5mL dropwise to a concentration of 1mol / L of acryloyl chloride solution, reacted in an ice-water bath for 30 minutes after dropping, then continued to react at room temperature for 2 hours and filtered, added 20 mL of deionized water to the filtered filtrate, precipitated solid precipitates, filtered, and used the filter cake Washing with ionized water until the pH value of the filtrate of the washed filter cake reaches neutral, vacuum-drying the filter cake to a constant weight, and pulverizing to obtain the acrylate functional monomer with a methylprednisolone structure. Wherein, the solution of acryloyl chloride is prepared by dissolving acryloyl chloride in dimethyl sulfoxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com