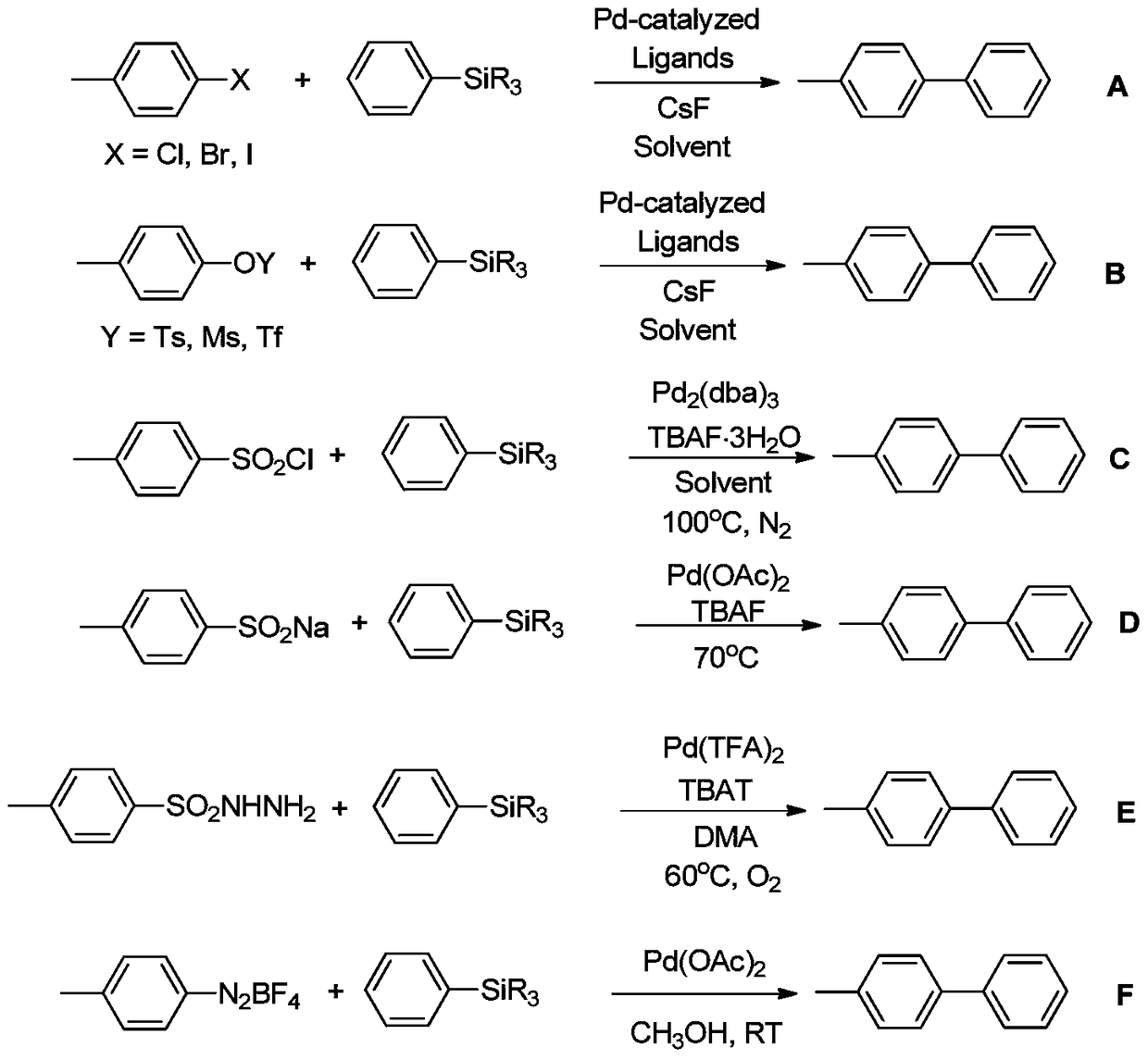
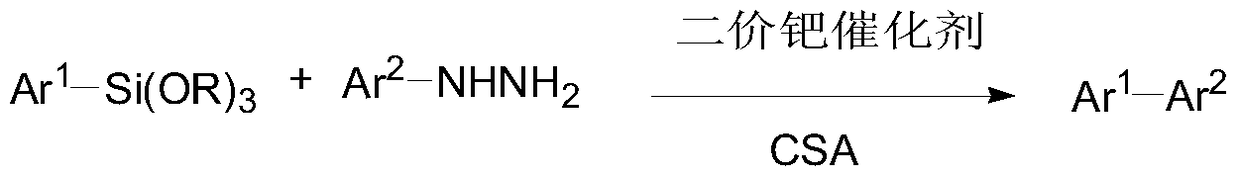
Process for preparing biaryls from aromatic hydrazines
A technology for the preparation of aromatic hydrazines and biaryls, applied in the field of aromatic hydrazines to prepare bisaryls, which can solve the problems of high reaction temperature of phenylhydrazine self-coupling side reactions, silane self-coupling side reactions cannot be ruled out, and the universality of the reaction has not been investigated. , to achieve high practicability and selectivity, inhibit self-coupling reaction, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~12
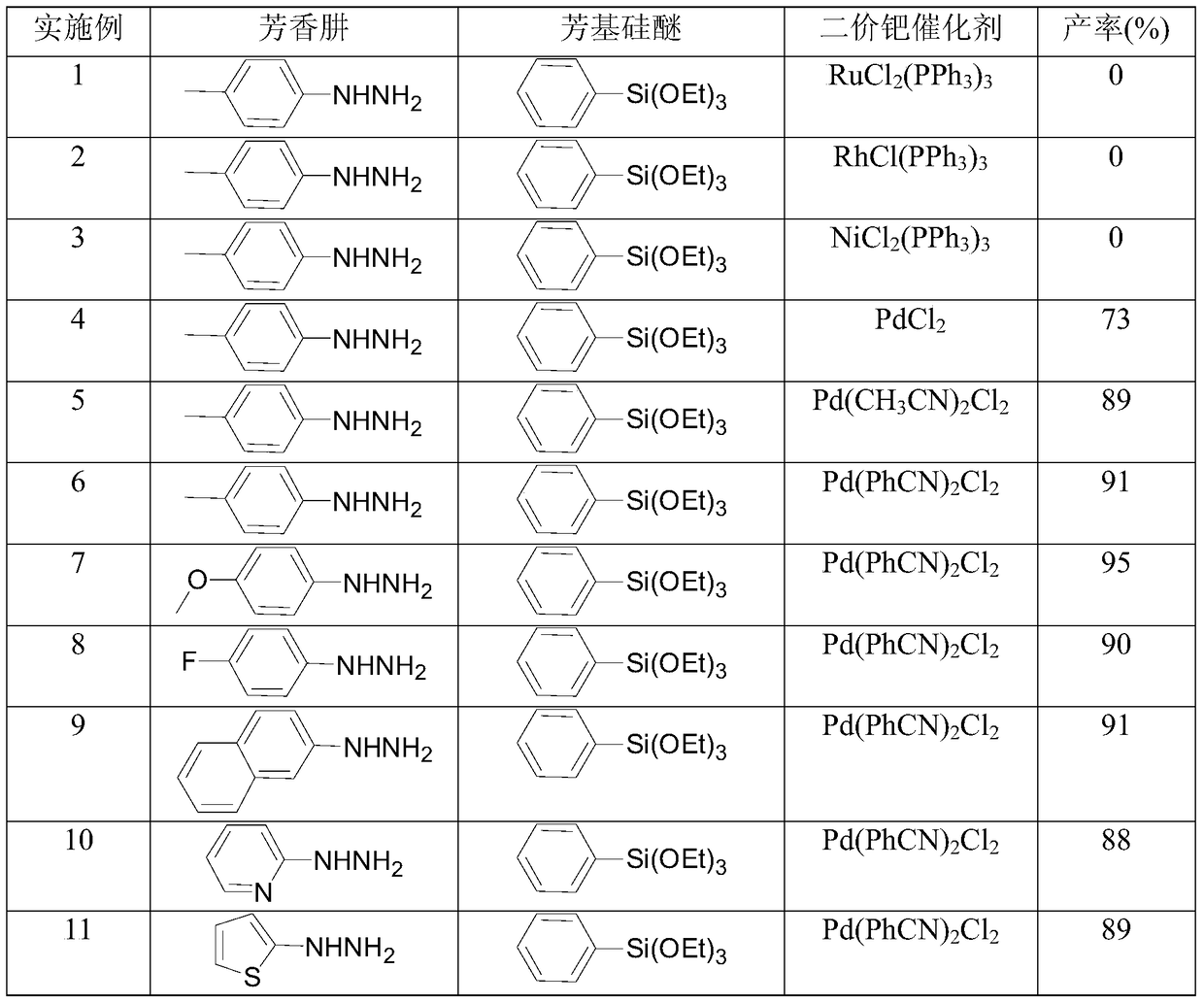
[0037] Add 1mmol of aromatic hydrazine, 1.2mmol of aryl silicon ether, 0.05mmol of divalent palladium catalyst and 1mmol of camphorsulfonic acid (CSA) into a round-bottomed flask containing 1ml of TBAF (1M) in THF, and stir at 50°C for 3 Hour. Cool down to room temperature after completion of the reaction, filter out the solid, spin dry the solvent, and separate by column to obtain the product. The raw materials, catalysts and reaction results used are shown in Table 1.
[0038] The used raw material and catalyst kind of table 1 embodiment 1~14
[0039]
[0040]
[0041] Some product characterization data are as follows:
[0042] 1-Phenylnaphthalene:
[0043]
[0044] White solid, m.p.41-42℃(lit. 2 mp 41-43°C); 1 H NMR (400MHz, CDCl 3 , TMS) δ7.91(t, J=8.0Hz, 1H), 7.59(d, J=8.0Hz, 2H), 7.48-7.54(m, 3H), 7.40-7.47(m, 5H), 7.32(t ,J=7.2Hz,1H). 13 C NMR (100MHz, CDCl 3 , TMS) δ141.06, 140.57, 134.10, 131.92, 130.31, 128.57, 127.94, 127.56, 127.23, 126.36, 126.01, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com