High efficiency water splitting oxygen electrode suitable for natural water and preparation method
A natural water body, oxygen electrode technology, applied in the direction of electrodes, electrode shapes/types, electrolytic components, etc., can solve the problems of high-efficiency and high-stable water splitting oxygen electrodes, etc., to achieve easy large-scale synthesis, high current density, and avoid side effects The effect of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Cobalt-iron hydrotalcite-conductive carbon black composite / titanium mesh water-splitting oxygen electrode for catalytic oxygen evolution in natural Bohai Sea water
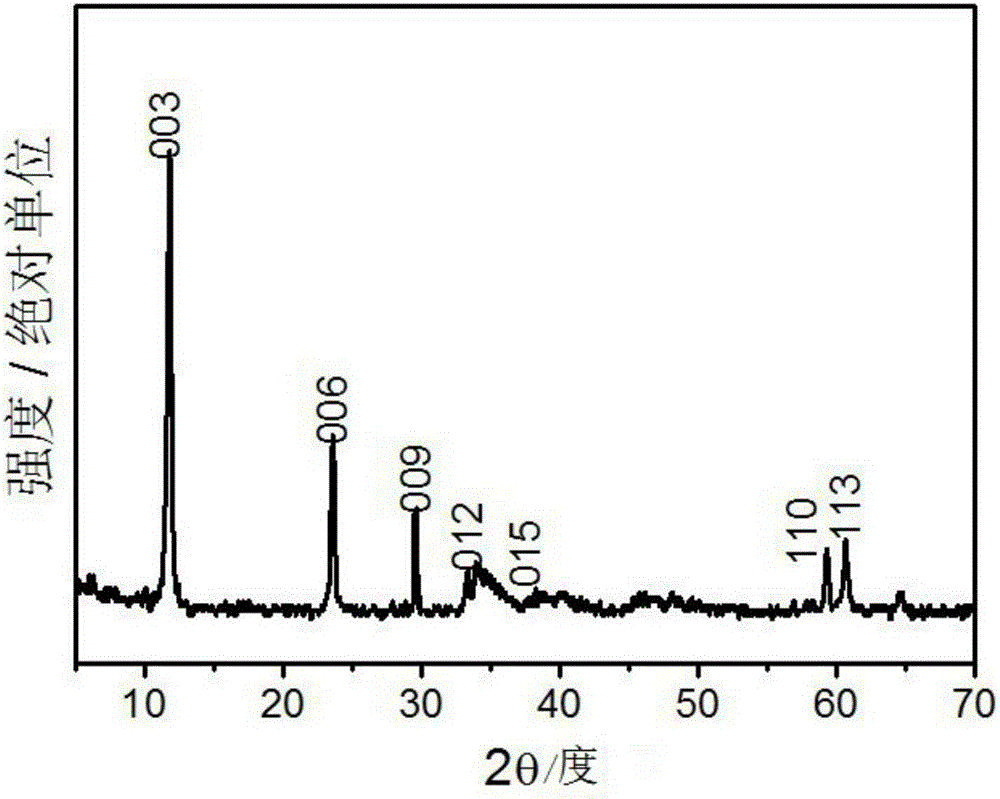
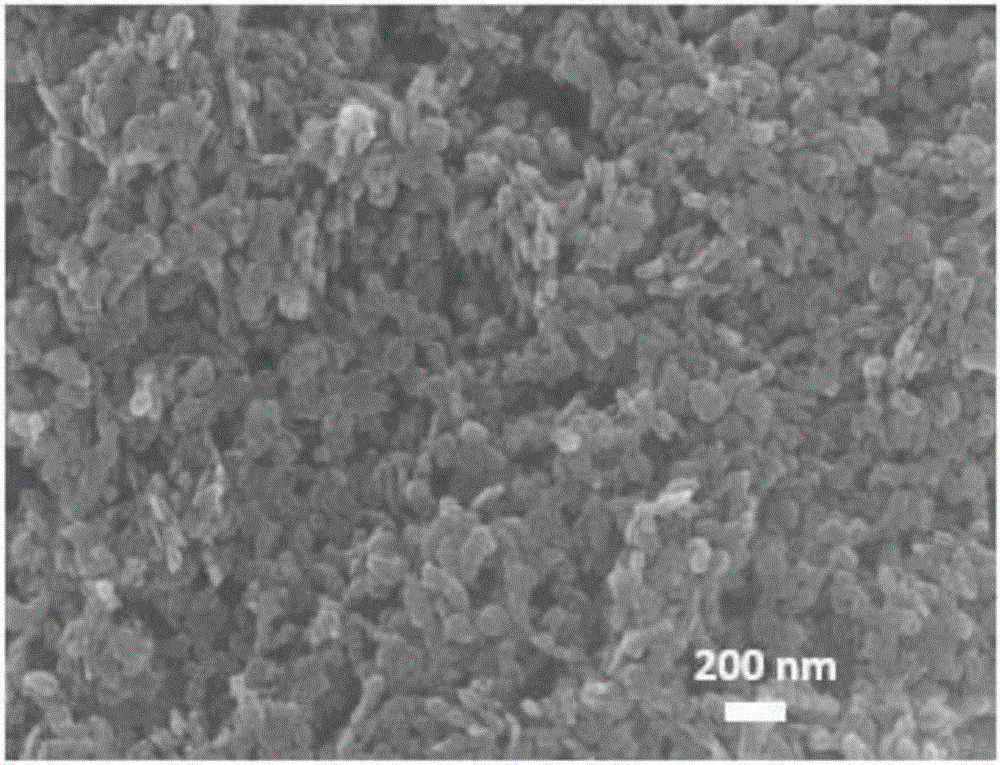
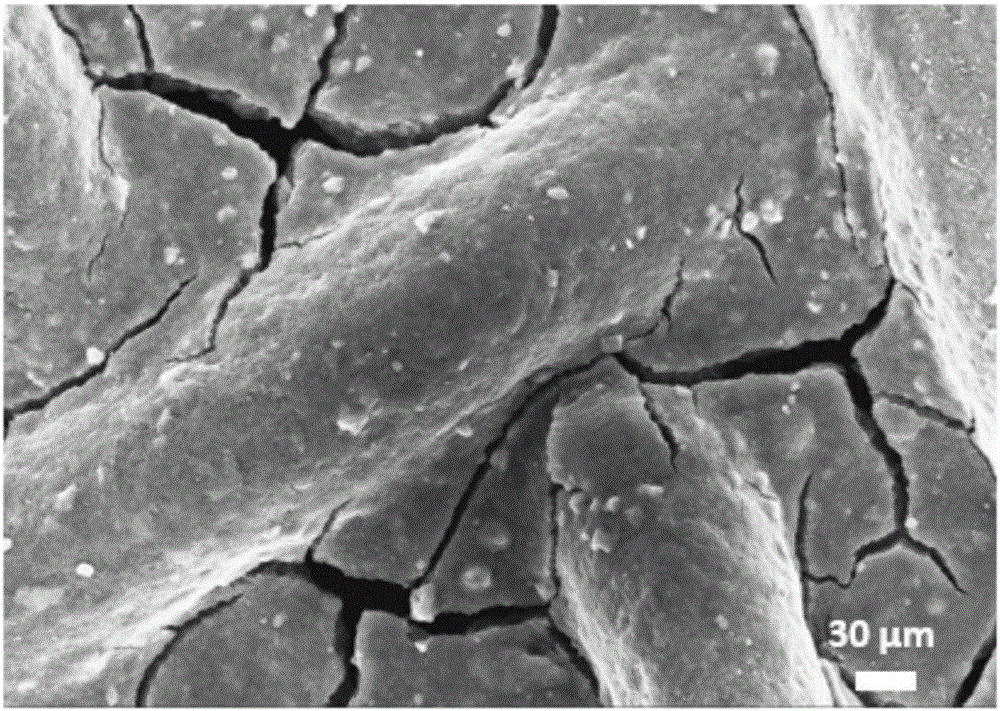
[0023] (1) Preparation of cobalt-iron hydrotalcite active material: weigh 11.636g Co(NO 3 ) 2 ·6H 2 O, pipette 8.076gFe (NO 3 ) 3 9H 2 O was dissolved in 100mL deionized water to make a mixed salt solution; then weighed 3.84gNaOH and 4.24gNaOH 2 CO 3 Dissolve in 100mL deionized water to make a mixed alkali solution; pour the above two solutions into a fully back-mixed rotary liquid film reactor and react for 2min at the same time, and the resulting slurry is crystallized in a water bath at 60°C for 12h to obtain a cobalt-iron hydrotalcite slurry, centrifuged Wash to pH = 7.5, dry at 80°C for 12 hours, and grind to obtain hydrotalcite nanoparticle powder, its XRD spectrum is as follows figure 1 As shown, the appearance of characteristic diffraction peaks such as (003), (006), and (012) indicates the g...
Embodiment 2
[0029] Cobalt-iron hydrotalcite-conductive carbon black composite / titanium mesh water-splitting oxygen electrode for catalytic oxygen evolution in artificial seawater
[0030] (1) Preparation of cobalt-iron hydrotalcite active material: weigh 17.454g Co(NO 3 ) 2 ·6H 2 O, pipette 8.076gFe (NO 3 ) 3 9H 2 O was dissolved in 100mL deionized water to make a mixed salt solution; then weighed 4.62gNaOH and 4.83gNaOH 2 CO 3 Dissolve in 100mL deionized water to make a mixed alkali solution; pour the above two solutions into a fully back-mixed rotary liquid film reactor and react for 2 minutes, and the resulting slurry is crystallized in a water bath at 50°C for 10 hours to obtain a cobalt-iron hydrotalcite slurry, centrifuged Wash to pH = 8, dry at 70° C. for 16 hours, and grind to obtain hydrotalcite nanoparticle powder.
[0031] (2) With the cobalt-iron hydrotalcite obtained in step (1) as the active material, after mixing CoFeLDH and conductive agent carbon black (VulcanXC-72...
Embodiment 3
[0035] Cobalt-iron hydrotalcite-conductive carbon black composite / titanium mesh water-splitting oxygen electrode for catalytic oxygen evolution in neutral sodium chloride solution
[0036] (1) Preparation of cobalt-iron hydrotalcite active material: weigh 14.16g Co(NO 3 ) 2 ·6H 2 O, pipette 9.11gFe (NO 3 ) 3 9H 2 O was dissolved in 100mL deionized water to make a mixed salt solution; then weighed 3.96gNaOH and 4.24gNaOH 2 CO 3 Dissolve in 100mL deionized water to make a mixed alkali solution; pour the above two solutions into a fully back-mixed rotating liquid film reactor at the same time and react for 1.5min, and the resulting slurry is crystallized in a water bath at 55°C for 5h to obtain a cobalt iron hydrotalcite slurry Centrifugal washing to pH = 7.5, drying at 60° C. for 24 hours, and grinding to obtain hydrotalcite nanoparticle powder.
[0037] (2) With the cobalt-iron hydrotalcite obtained in step (1) as the active material, after mixing CoFeLDH and conductive ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com