A kind of crystal form of cyclopropanecarboxamide derivative and preparation method thereof
A technology of cyclopropanecarboxamide and derivatives, which is applied in organic chemistry methods, drug combinations, bone diseases, etc., can solve problems such as instability and unfavorable hygroscopicity of cyclopropanecarboxamide derivatives, and achieve excellent high temperature stability, Good bioavailability, low hygroscopic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 15
[0033] Preparation of Embodiment 1 to 15 Cyclopropanecarboxamide Derivative A Crystal Form
[0034] Weigh 500 mg of the cyclopropanecarboxamide derivative raw material into a container, add 100 ml of the solvent (analytical grade) in Table 1, suspend at 35° C. for 48 hours, filter, and vacuum-dry to obtain off-white powder. Weigh to calculate its yield.
[0035] Table 1 Preparation of crystal form A of cyclopropanecarboxamide derivatives
[0036] Example solvent yield 1 n-propanol 76% 2 Isopropanol 78% 3 sec-butanol 79% 4 Butyl acetate 66% 5 n-heptane 86% 6 n-Hexane 85% 7 Cyclohexane 88% 8 acetone 73% 9 Ether 88% 10 isopropyl ether 77% 11 methyl tert-butyl ether 76% 12 toluene 86% 13 p-xylene 84% 14 Tetrahydrofuran 76% 15 Dichloromethane 76%
Embodiment 16
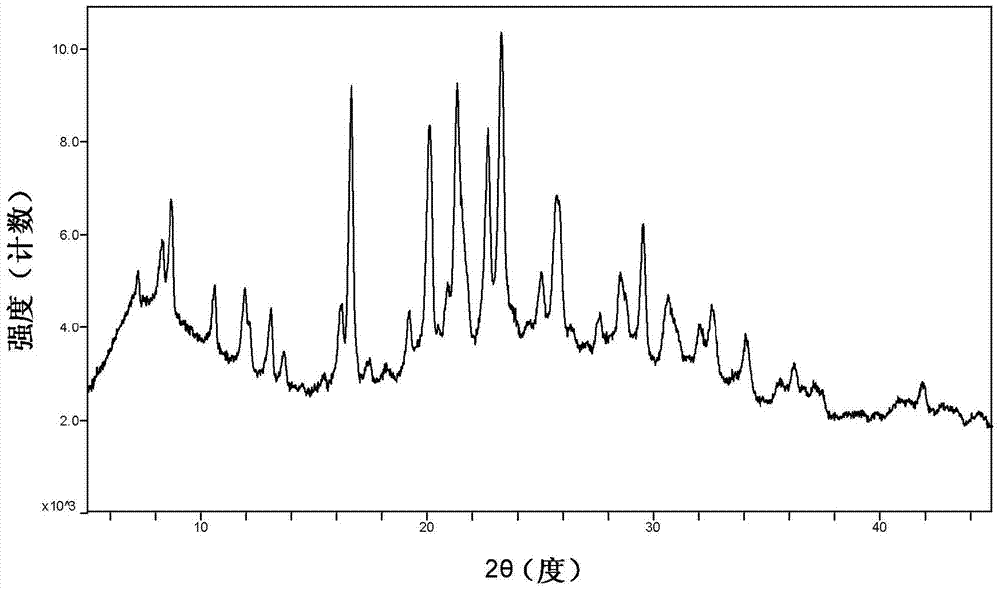
[0037] Example 16. Characterization of cyclopropanecarboxamide derivative A crystal form by XRPD pattern
[0038] The measurement of the X-ray powder diffraction (XRPD) pattern is carried out using the Rigaku UltimaIV model combined multifunctional X-ray diffractometer, and the specific collection information is as follows: Cu anode (40kV, 40mA), scanning speed 20° / min, scanning range (2θ range )3~45°, scan step size 0.02, slit width 0.01. Samples were processed using glass slides pressed directly onto the test plate. Subsequent XRPD patterns all adopt similar measurement methods.
[0039]Determination of the XRPD spectrum of the cyclopropanecarboxamide derivative A crystal form prepared according to the method described in Example 1, at 2θ=8.3, 8.702, 10.62, 11.94, 13.101, 13.699, 16.217, 16.659, 19.218, 20.1, 20.939, 21.34, There are diffraction peaks at 21.597, 22.72, 23.32, 25.098, 25.841, 27.62, 28.542, 29.541, 30.739, 32.06, 32.6, 34.14, 35.621, 36.261, 37.12, and 37.5...
Embodiment 17
[0041] Example 17. Investigation of High Temperature Stability of Crystal Form A of Cyclopropanecarboxamide Derivatives
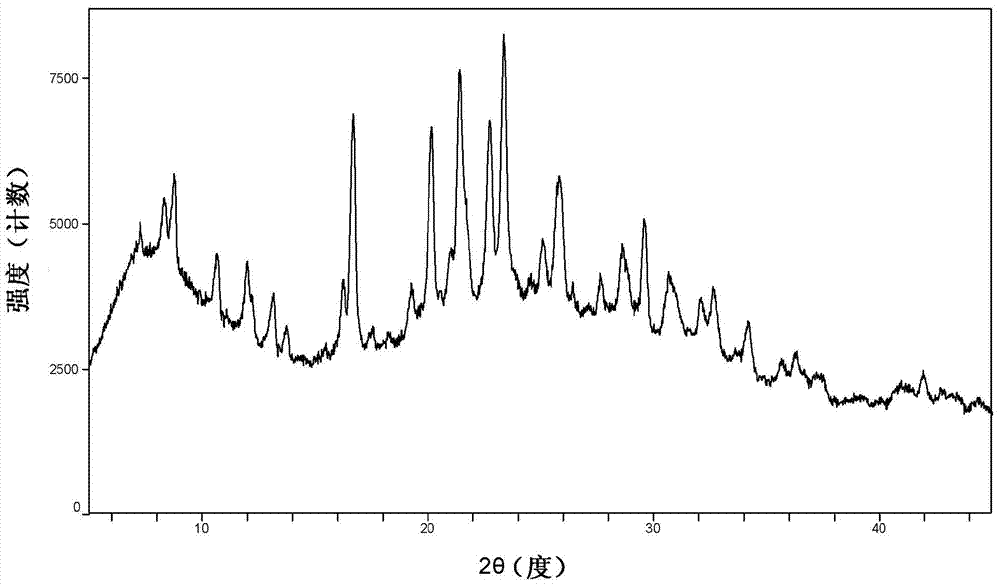
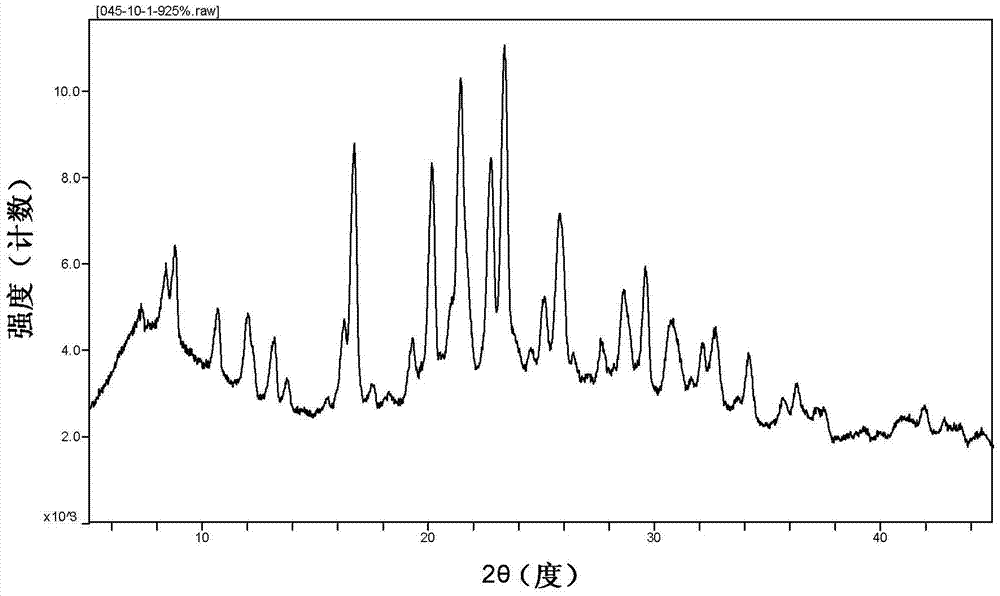
[0042] The cyclopropanecarboxamide derivative A crystal sample was placed in a 60°C oven, and after 5 days and 10 days, the sample was taken out for XRPD testing (such as figure 2 and Figure 5 Shown), in order to investigate the stability of the crystal form of the sample to temperature. The results show that the crystal form A sample is stable under high temperature conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water solubility | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com