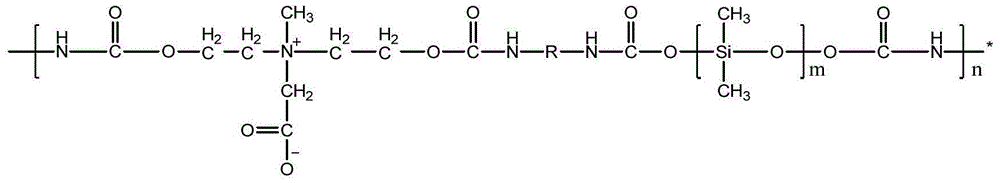
Amphipathic polyurethane with anti-bacterial and anti-protein function as well as preparation method and application of amphipathic polyurethane
A technology of amphiphilic polyurethane, which is applied in the field of amphiphilic polyurethane and its preparation, can solve the problems of shortened service life and decreased stability of materials, and achieve good biological stability, biocompatibility and excellent antifouling effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of amphiphilic polyurethane
[0024] (1) Under a nitrogen atmosphere, add 15g of hydroxypolydimethylsiloxane, 3.6g of hexamethylene diisocyanate, and 43.4g of N,N-dimethylformamide into a 200mL three-necked flask at a rate of *0.022*g In the case of dibutyltin laurate as a catalyst, react at 60° C. for 8 hours to obtain an isocyanate-terminated prepolymer.
[0025] (2) Dissolve 3.36g of quaternary ammonium dihydric alcohol in 7.85g of N,N-dimethylformamide, and add it dropwise to the above prepolymer solution, react at 60°C for 8h, The target polyurethane product was obtained.
[0026] (3) Hydrolyze the coating with a mixed solution of trifluoroacetic acid and dichloromethane (volume ratio 1:1) for 1 to 2 hours, and then neutralize it with saturated sodium bicarbonate solution to obtain amphiphilic polyurethane.
[0027] Structural characterization of the obtained amphiphilic polyurethane was carried out by Fourier transform infrared spec...
Embodiment 2
[0029] Embodiment 2: the preparation of amphiphilic polyurethane
[0030] (1) Under a nitrogen atmosphere, add 10g of hydroxypolydimethylsiloxane, 6g of hexamethylene diisocyanate, and 37.3g of N,N-dimethylformamide into a 100mL three-necked flask, and dilute with *0.02*g In the case of dibutyltin laurate as a catalyst, react at 100° C. for 3 hours to obtain an isocyanate-terminated prepolymer.
[0031] (2) Dissolve 4g of quaternary ammonium salt dihydric alcohol in 9.3g of N,N-dimethylformamide, and add it dropwise to the above prepolymer solution, and react at 100°C for 3h to obtain Target polyurethane product.
[0032] (3) The coating was hydrolyzed with a mixed solution of trifluoroacetic acid and dichloromethane (volume ratio 1:5) for 1-2 hours, and then neutralized with saturated sodium bicarbonate solution to obtain amphiphilic polyurethane.
[0033] Structural characterization of the obtained amphiphilic polyurethane was carried out by Fourier transform infrared spec...
Embodiment 3
[0035] Embodiment 3: the preparation of polysiloxane type polyurethane coating
[0036] (1) Under a nitrogen atmosphere, add 31.5g of hydroxy polydimethylsiloxane, 5.04g of hexamethylene diisocyanate, and 85.26g of N,N-dimethylformamide into a 200mL three-necked flask, with a concentration of *0.037* g dibutyltin dilaurate as a catalyst, react at 70° C. for 3 hours to obtain an isocyanate-terminated prepolymer.
[0037] (2) Add 4 g of poly[(isocyanate phenyl)-co-formaldehyde] and 3 drops of stannous octoate to the solution for further cross-linking.
[0038] (3) Cast the solution onto a polytetrafluoroethylene template, and cure it in a vacuum oven at 80° C. for 24 hours to obtain a polysiloxane polyurethane coating.
[0039] (4) Hydrolyze the coating with a mixed solution of trifluoroacetic acid and dichloromethane (volume ratio 1:2) for 1 to 2 hours, and then neutralize it with a saturated sodium bicarbonate solution to obtain an amphiphilic polyurethane coating.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com