Fluorescent molecular probe and application thereof in detecting carbohydrates
A technology of fluorescent molecular probes and carbohydrate substances, which is applied in fluorescence/phosphorescence, luminescent materials, material excitation analysis, etc., to achieve the effects of sensitive fluorescence response, short reaction time, and strong anti-interference ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1Q1
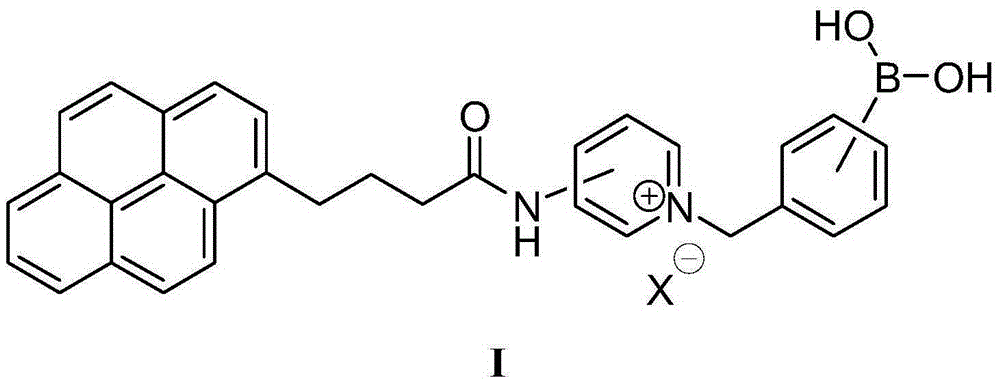
[0066] The preparation of fluorescent molecular probe shown in embodiment 1Q1
[0067]
[0068] The synthesis of the fluorescent molecular probe shown in Q1 is divided into two steps:
[0069] first step,
[0070]
[0071] 1-pyrenebutyric acid (0.288g, 1mmol), WSC·HCl (0.211g, 1.1mmol) and HOBt (0.149g, 1.1mmol) were dissolved in 10mL of DMF, and stirred in an ice bath for 5 minutes. 3-Aminopyridine (0.094 g, 1 mmol) was added to the reaction solution and stirred overnight. 50 mL of dichloromethane was poured into the reactant, washed successively with hydrochloric acid (0.1M, 30 mL), water (2×30 mL) and saturated sodium chloride solution (30 mL). The organic solvent dichloromethane was removed with a rotary evaporator to obtain a yellow crude product, which was recrystallized with chloroform and cyclohexane to obtain an intermediate product Qm (0.22 g, 60%).
[0072] second step,
[0073]
[0074] The intermediate product Qm (0.182g, 0.5mmol) and 4-(bromomethyl)p...
Embodiment 2Q1
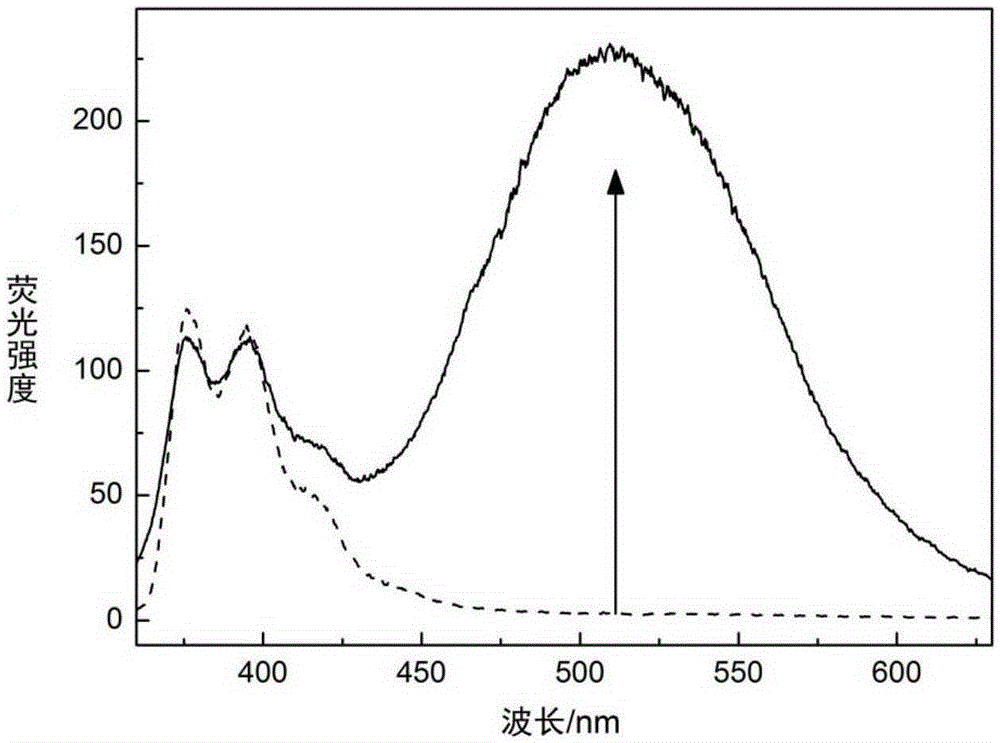
[0076] Fluorescence spectra before and after the fluorescent molecular probe shown in embodiment 2Q1 is combined with glucose
[0077] To prepare the probe solution:
[0078] Prepare 1000mL of 50mmol / LpH10.0 carbonate buffer. Accurately weigh 0.029g of Q1, dissolve it in 10mL of methanol, and prepare Q1 mother liquor 0.005mol / L. Pipette 2 mL of the mother liquor and add it to 98 mL of carbonic acid buffer to obtain a 0.1 mmol / L probe solution containing the fluorescent molecular probe indicated by Q1.
[0079] Prepare 1mol / L glucose solution.
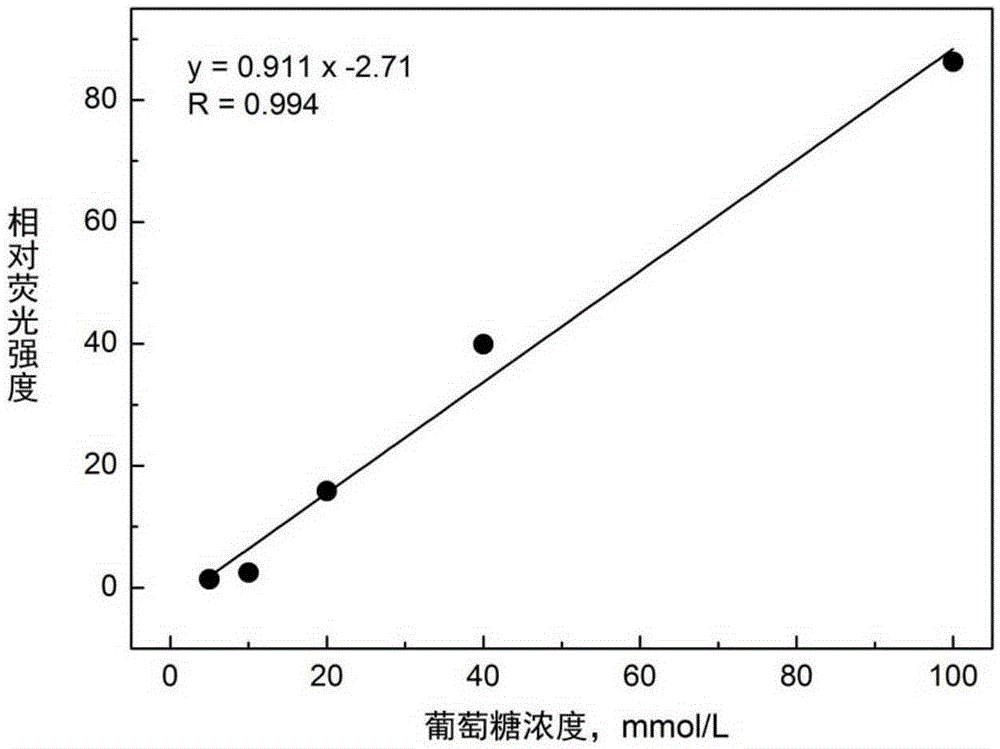
[0080] Pipette 2mL of the probe solution into a fluorescence cuvette, draw a spectrum with a fluorescence spectrophotometer, and the excitation wavelength is 377nm. Then, 20 μL of glucose solution was added dropwise to 2 mL of the probe solution, stirred evenly, and a spectrogram was drawn with a fluorescence spectrophotometer, and the excitation wavelength was 377 nm. Comparison of fluorescence spectra emitted by fluorescent molecu...
Embodiment 3
[0083] The analysis of embodiment 3 tobacco water-soluble total sugars
[0084] 1) Sample pretreatment
[0085] Prepare samples according to the method described in YC / T31-1996 "Preparation of Tobacco and Tobacco Products Samples and Oven Method for Moisture Determination", and measure the moisture content.
[0086] Weigh 0.5g sample, add 20mL 5v / v% acetic acid solution, cover the stopper, shake and extract on the shaker for 30min. Filter with qualitative filter paper, collect the filtrate and mix evenly with 10mL of 1.0mol / L hydrochloric acid, heat in a water bath at 95°C for 15min, cool and then neutralize with 1.0mol / L sodium hydroxide to a pH value of 6.5-7, and then fix with deionized water The volume was reduced to 50mL, and the obtained sample extract was to be tested.
[0087] 2) Preparation of probe solution
[0088] Prepare 1000mL of 50mmol / LpH10.0 carbonate buffer. Accurately weigh 0.029g of Q1, dissolve it in 10mL of methanol, and make Q1 mother liquor 0.005mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com