Method of avian infectious laryngotracheitis live vaccine using cell line
A technology of tracheitis and cell lines, applied in biochemical equipment and methods, microorganisms, pharmaceutical formulas, etc., can solve the problems of SPF embryo purity, chickens are prone to latent infection, and users cannot be obtained, so as to reduce immune side effects Response problems, reduced batch-to-batch variability, and clear-cut effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Screening of virus-adapted cells
[0033] 1 selection of cells
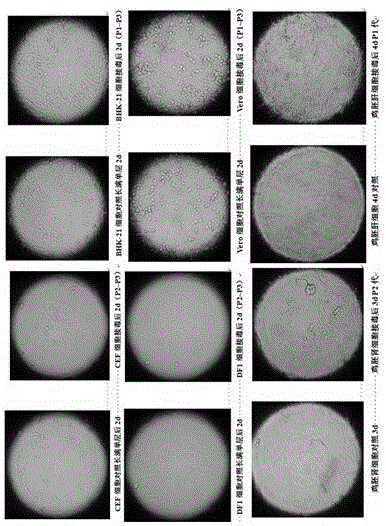
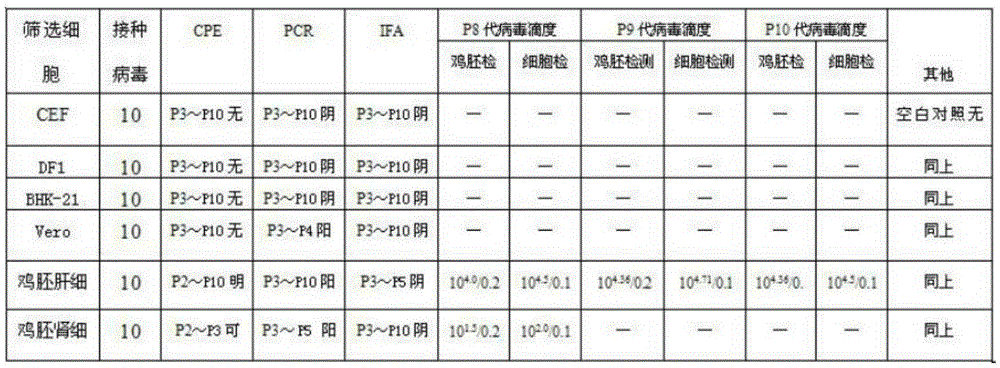
[0034] AILTV and HSV belong to the α subfamily of Herpesvirus genus, and their characteristics have many similarities. According to relevant reports, HSV can grow well in various cells such as BHK cells and Vero cells. For screening AILTV adaptive cells, the present invention selects BHK-21 and Vero cells were used for propagation of AILTV. The selection of DF1, primary CEF cells, chicken embryonic liver and kidney cells is mainly based on the strong culture characteristics of ILTV host specificity.
[0035] 2 Preparation and passage of cells
[0036] CEF, chicken embryo liver, chicken embryo kidney three kinds of cells are chicken embryo primary cell, use respectively the whole chicken embryo that removes the eyeball, the shredded chicken embryo liver, the smashed chicken embryo kidney through 2.5% trypsin (100 units / ml double antibody, pH 7.5-7.8) was digested, and prepared by adding M199 g...
Embodiment 2
[0046] Example 2 Cloning of Chicken Embryo Hepatocytes
[0047] 1 Cloning of chicken embryo liver cells
[0048] 1.1 Selection of chicken embryo liver cells
[0049] Take the prepared chicken embryo liver cells, count the cells, inoculate them in a cell bottle with a cell number of 3 million / ml, shake well, place them in a 5% CO2 incubator for 10 minutes, discard the culture medium, and add 12 % fetal bovine serum M199 growth medium, reset in the incubator and cultivated until monolayer cells.
[0050] 1.2 Chicken embryo liver cell cloning
[0051] Trypsinize and resuspend the chicken embryonic hepatocytes covered with a single layer in step 1.1, add 8% to 12% fetal bovine serum M199 growth medium for dilution, the dilution concentration is 2 cells / ml, and mix well , add to a 48-well cell plate, 0.5ml / well, place in a 5% CO2 incubator for 10 minutes after adsorption, discard the culture medium, add 1ml of growth medium / well, and record the content of Cultivate for 1 to 2 day...
Embodiment 3
[0084] Embodiment 3 chicken infectious laryngotracheitis virus clone purification
[0085] 1 Experimental materials
[0086] Agar powder, cell maintenance solution, 6-well cell culture plate, seed virus of AILTVK317 strain.
[0087] 2 Experimental methods
[0088] 2.1 Selection of virus dilution factor
[0089] Take the seed virus of AILTVK317 strain, use the cloned chicken embryo liver cells to measure the virus titer, and carry out 10-fold serial dilution of the virus according to the titer test results, and dilute to 10 -8 , take 10 -4 、10 -5 、10 -6 、10 -7 、10 -8 Virus dilutions were inoculated into 6-well cell plates full of monolayer cells, and three replicate cell wells were inoculated for each dilution factor, adsorbed in a 5% CO2 incubator for 1.5 hours, discarded the adsorption solution, and washed with 1×PBS (PH is 7.2) wash 2 times, add 0.5-1% agar powder cell maintenance solution, place it upside down in the incubator for culture, after 4-5 days, select dil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com