A kind of porcine circovirus type 2 antigen purification and concentration method
A porcine circovirus and antigen technology, applied in the field of biomedicine, can solve the problems of high cost of purification and concentration, poor immune protection effect of vaccines, and insignificant effect of antigen titer, so as to achieve high antigen recovery efficiency, reduce immune side effects, and improve Effects on Vaccine Safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
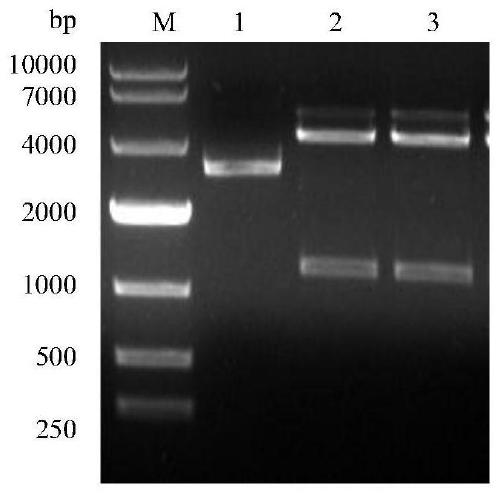
[0027] Example 1 Construction and Identification of Recombinant Expression Plasmid pET-PL
[0028] Design the adapter protein, the amino acid sequence of the adapter protein is shown in SEQ ID NO: 1, the protein is a fusion protein, the fusion protein contains two functional recognition regions, which can specifically recognize the PCV2 antigen and the Lactococcus lactis backbone respectively, the linker The gene encoding the protein is shown in SEQ ID NO:2. In order to facilitate the identification of the adapter protein, a His tag protein (HHHHHH) was added to the carboxyl terminus of the sequence shown in SEQ ID NO: 1, and the His tag protein coding sequence was added before the stop codon of the adapter protein coding gene to obtain the sequence SEQ ID NO: 3, In addition, an Nde I restriction site was designed at the 5' end, and a HindIII restriction site was designed at the 3' end respectively, which were sent to GenScript for synthesis. Insert the synthesized gene fragm...
Embodiment 2
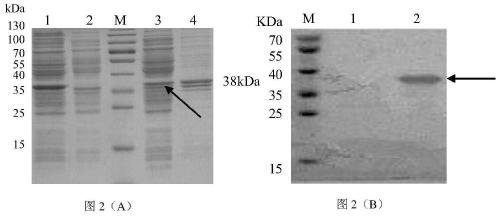
[0038] Example 2 Construction of Recombinant Expression Bacteria PL / BL21 and Expression of Adapter Protein
[0039] 1. Construction of recombinant expression strain PL / BL21
[0040]The recombinant plasmid pET-PL obtained in Example 1 was transformed into the prokaryotic expression strain BL21(DE3) to obtain the recombinant expression strain PL / BL21. At the same time, an empty pET32a plasmid was set as a control to transform BL21 (DE3) to obtain a control strain pET / BL21.
[0041] 2. Induced expression and identification of recombinant expression strain PL / BL21
[0042] (1) pick the single clones of bacterial strain PL / BL21 and control strain pET / BL21, respectively inoculate them into LB liquid medium containing 50 mg / mL ampicillin, and culture them overnight at 37°C and 200rmp to obtain mother liquor;
[0043] (2) Inoculate the mother liquor of each of the above strains into fresh LB liquid medium (containing 50 mg / mL ampicillin) at a ratio of 1:200, culture at 37°C and 180r...
Embodiment 3
[0053] Example 3 Adapter protein quantitative analysis
[0054] 1. Preparation of Purification Vector
[0055] Lactococcus lactis IL1403 (The Complete Genome Sequence of the Lactic Acid Bacterium Lactococcus lactis ssp.lactisIL1403, Genome Res., 10.1101 / gr.169701) was cultured statically at 30°C with fresh GM17 medium, and the culture time was 16-18h. Centrifuge the culture solution at 8000rpm for 5min, collect the bacteria, wash the precipitate with PBS buffer once, add 0.1M hydrochloric acid to boil for 30min, centrifuge at 8000rpm for 5min, then wash the precipitate with PBS buffer for 3 times, and finally buffer with PBS solution to obtain the Lactococcus lactis skeleton, which is the purified carrier, and the hemocytocyte count of the purified carrier was obtained, and 2.5×10 9 A purified carrier is recorded as 1 unit.
[0056] 2. Protein Dissociation
[0057] The adapter protein solution was prepared according to the method in Example 2. In 2mL adapter protein soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com