Solubilization systems, solid dispersions and pharmaceutical preparations
A solid dispersion and system technology, which can be used in pharmaceutical combinations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of complex process, low preparation formability, low solubility, etc. The effect of specific surface area, improvement of hydrophilic properties, and improvement of solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
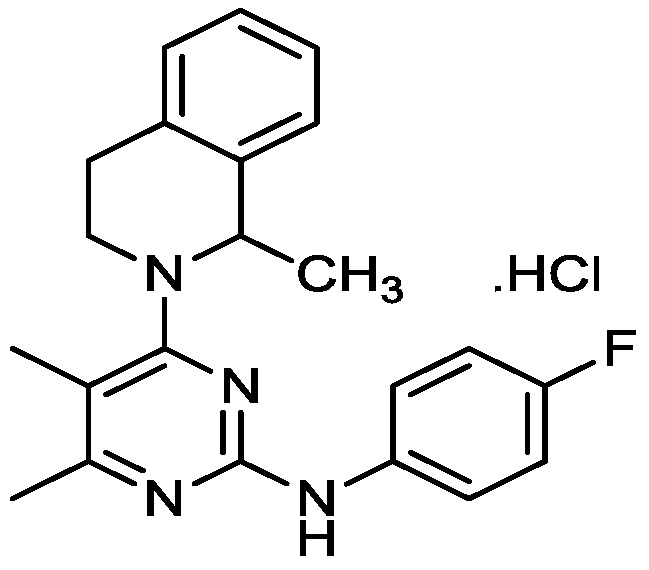
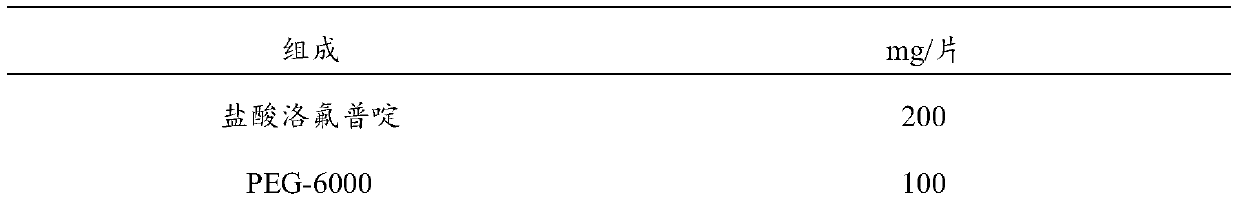
[0035] Tablets with a content of 200mg / tablet of loflupridine hydrochloride are composed as shown in Table 1.
[0036] Table 1 Composition of Loflupridine Hydrochloride Tablets
[0037]
[0038]
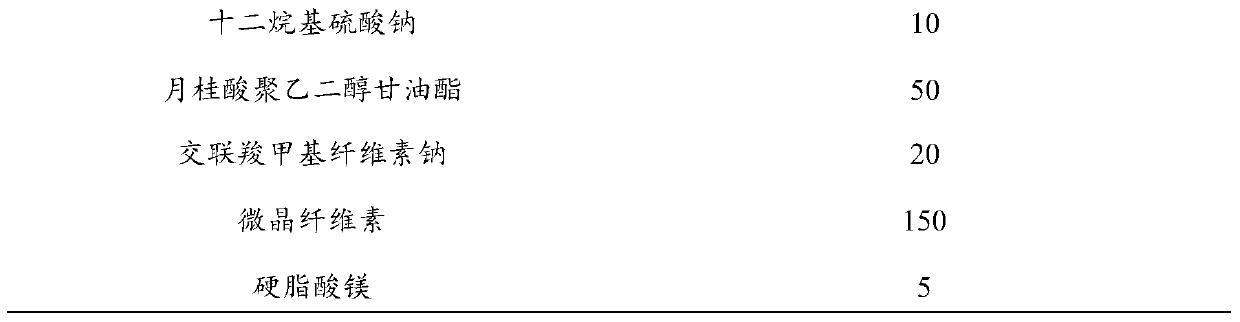
[0039] 1.1 Mix 200g of loflupridine hydrochloride, 100g of polyethylene glycol-6000, 10g of sodium lauryl sulfate and 50g of macrogolglycerol laurate, heat and melt it into a white suspension to obtain the first mid product.
[0040] 1.2 Mix 150 g of microcrystalline cellulose and 20 g of croscarmellose sodium evenly, add the first intermediate product, wet granulate, dry, granulate, add magnesium stearate, mix, and tablet to obtain tablets.
Embodiment 2
[0042] The composition of the capsules with a loflupridine hydrochloride content of 200mg / capsules is shown in Table 2.
[0043] Table 2 Composition of Loflupridine Hydrochloride Capsules
[0044]
[0045] 2.1 Dissolve 200g of PEG-4000 in 50g of purified water, add 5g of sodium lauryl sulfate and stir to dissolve, then add 20g of macrogol glycerol stearate, stir to dissolve, and add 200g of loflupridine hydrochloride at 60°C The water bath was stirred at least 1000 rpm to make it homogenized, and the first intermediate product was obtained.
[0046] 2.2 Add 100g of mannitol and 10g of crospovidone into the fluidized bed, then spray into the first intermediate product obtained in 2.1, granulate in the fluidized bed, pass through a 20-mesh sieve for granulation, add 5g of silicon dioxide, and mix Evenly, put it into capsules.
Embodiment 3
[0048] The composition of the granule of loflupridine hydrochloride content 100g / bag is as shown in table 3.
[0049] Table 3 Composition of Loflupridine Hydrochloride Granules
[0050]
[0051] Dissolve 300g of povidone K30 in 1000g of water, add 5g of sodium lauryl sulfate and 10g of macrogolglycerol laurate and stir to dissolve, then add 100g of loflupridine hydrochloride and stir at high speed to fully suspend , using a spray drying method to prepare granules, add 5g of magnesium stearate, and pack in sachets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com