Photosensitizer phospholipid compound as well as pharmaceutical composition and application of photosensitizer phospholipid compound
A technology of photosensitizer phospholipids and phospholipid compounds, which is applied in the field of medicine, can solve the problems of high price, drug leakage, and difficulty in drug efficacy, and achieve strong phototoxicity, low dark toxicity, and overcome the effect of phototoxicity reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
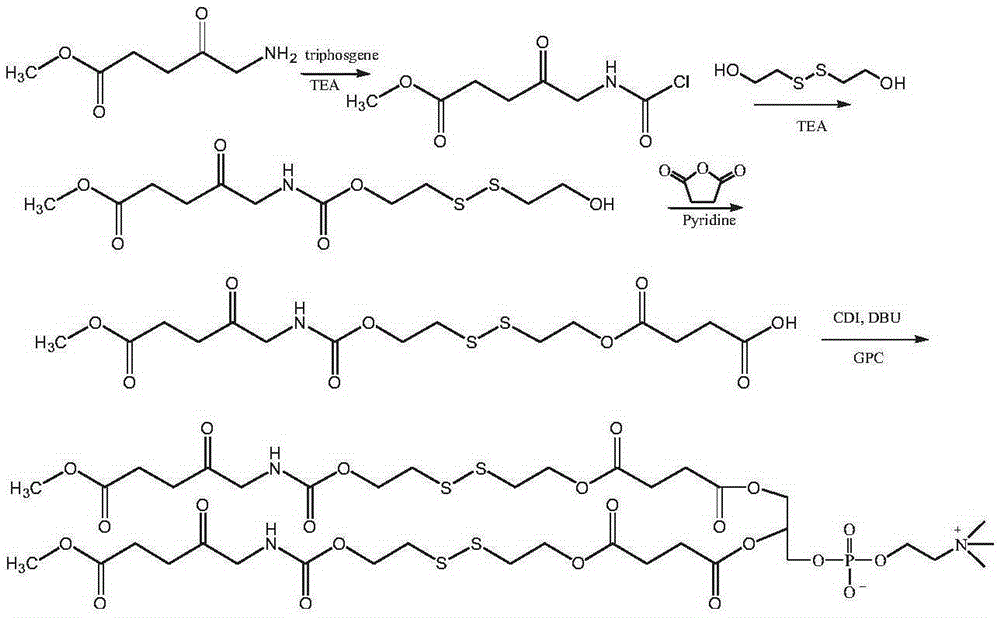
[0089] The synthesis of bis(methoxycarbonyl propionyl methylene carbamate-dithiodiethylene glycol-succinate) phosphatidylcholine compound (see the synthetic route figure 1 )
[0090] Dissolve 1g of methyl aminolevulinate in 20mL of chloroform, add 0.6g of triethylamine and 0.3g of triphosgene, react at room temperature for 6h, remove the solvent by rotary evaporation, dissolve the solid in 10mL of DMSO, add 0.3g of triethylamine, add 0.6 g of dithiodiethylene glycol was reacted at room temperature for 24 hours, and the resulting reaction solution was purified by column chromatography to obtain 0.31 g of methoxycarbonylpropionylmethylenecarbamate-dithiodiethylene glycol. Methoxycarbonylpropionylmethylenecarbamate-dithiodiethylene glycol, 2g of succinic anhydride dissolved in 30mL of pyridine, reacted at 40°C for 48h; the solvent was removed by rotary evaporation, the precipitate was precipitated in cold ether, washed with dilute hydrochloric acid, 0.81 g of the intermediate pr...
Embodiment 2
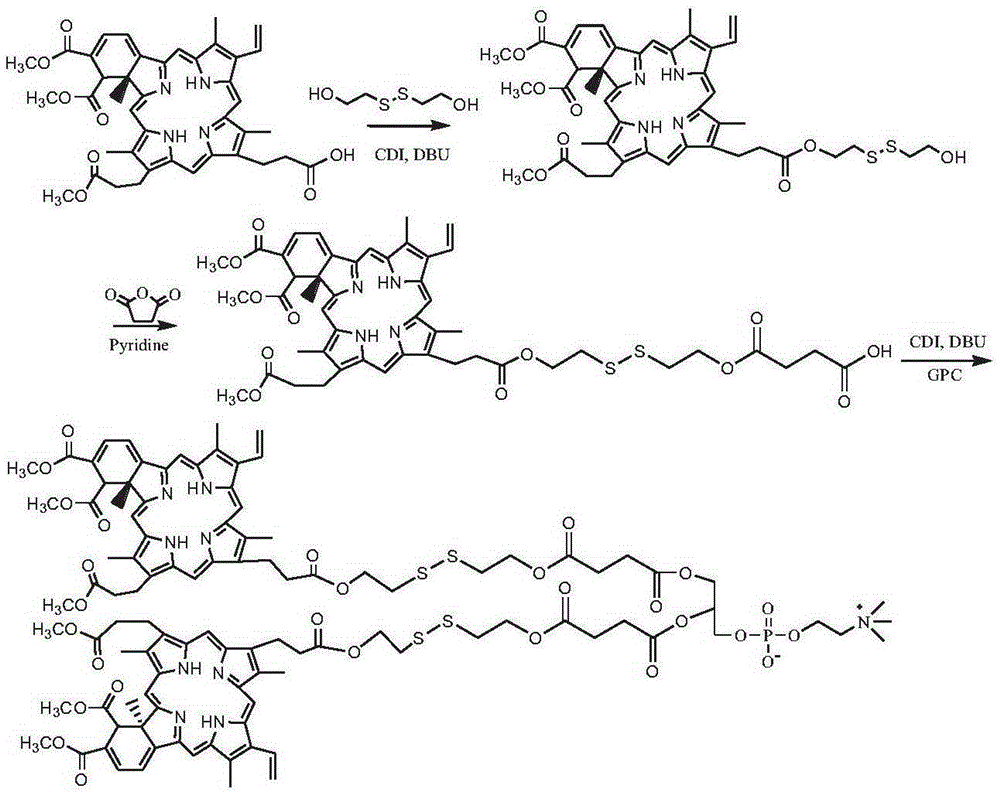
[0093] Synthesis of two (verteporfin-dithiodiethylene glycol-succinate) phosphatidylcholine compounds (see the synthetic route figure 2 )
[0094] Dissolve 1 g of Verteporfin in 20 mL of chloroform, add 0.5 g of CDI, activate for 1 h, add 0.6 g of dithiodiethylene glycol, 0.5 g of DBU, react at room temperature for 24 h, and the resulting reaction solution is passed through column chromatography After purification, 0.31 g of verteporfin-dithiodiethylene glycol monoester was obtained. Verteporfin-dithiodiethylene glycol monoester, 2 g of succinic anhydride dissolved in 30 mL of pyridine, reacted at 40 ° C for 48 h; the solvent was removed by rotary evaporation, the precipitate was precipitated in cold ether, washed with dilute hydrochloric acid, and the intermediate product Vertepor was obtained Fen-dithiodiethylene glycol succinate monoester 0.81g. The intermediate product verteporfin-dithiodiethylene glycol succinic acid monoester 0.8g was dissolved in 20mLDMSO, added CDI0...
Embodiment 3
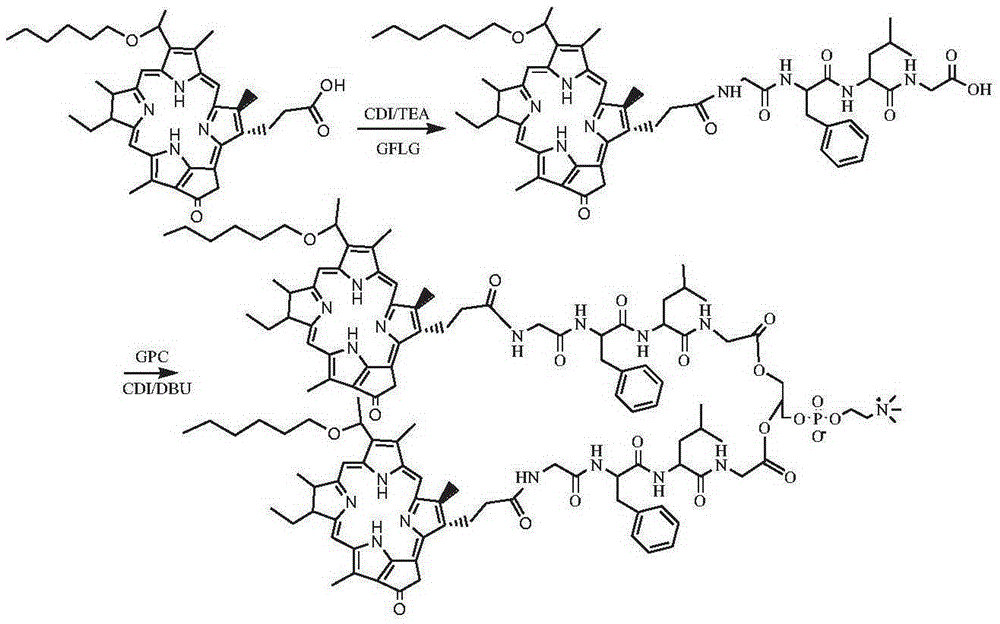
[0097] The synthesis of two (HPPH-GFLG) phosphatidylcholine compounds (the synthetic route sees image 3 )
[0098] Dissolve 1 g of photochlor (HPPH) in 20 mL of DMF, add 0.5 g of CDI and 0.5 g of TEA, activate for 1 h, then add 0.5 g of N-glycyl-phenylalanyl-leucyl-glycine (GFLG) and react at room temperature After 24 hours, the reaction solution was separated by column chromatography to obtain 0.65 g of the intermediate product HPPH-GFLG. The product was dissolved in 15 mL of dimethyl sulfoxide, added with 0.6 g of CDI, 0.3 g of GPC and 0.6 g of DBU, reacted at room temperature for 24 hours, precipitated in cold ether, and separated by column chromatography to obtain bis(HPPH-GFLG) phosphatidylcholine 0.45 g. 1 HNMR (500MHz, CD 3 OD: CDCl 3 1:1): δ8.01, 7.21, 7.12, 7.08, 4.92, 4.64, 4.53, 4.32, 4.16, 4.09, 3.97, 3.77, 3.70, 3.43, 3.37, 3.30, 3.07, 3.05, 2.24, 2.22, 2.05, 1.83 , 1.75, 1.71, 1.46, 1.33, 1.31, 1.29, 1.01, 0.96. [M+H] + m / z, 2244.71.
[0099] Bis(HPPH-GF...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com