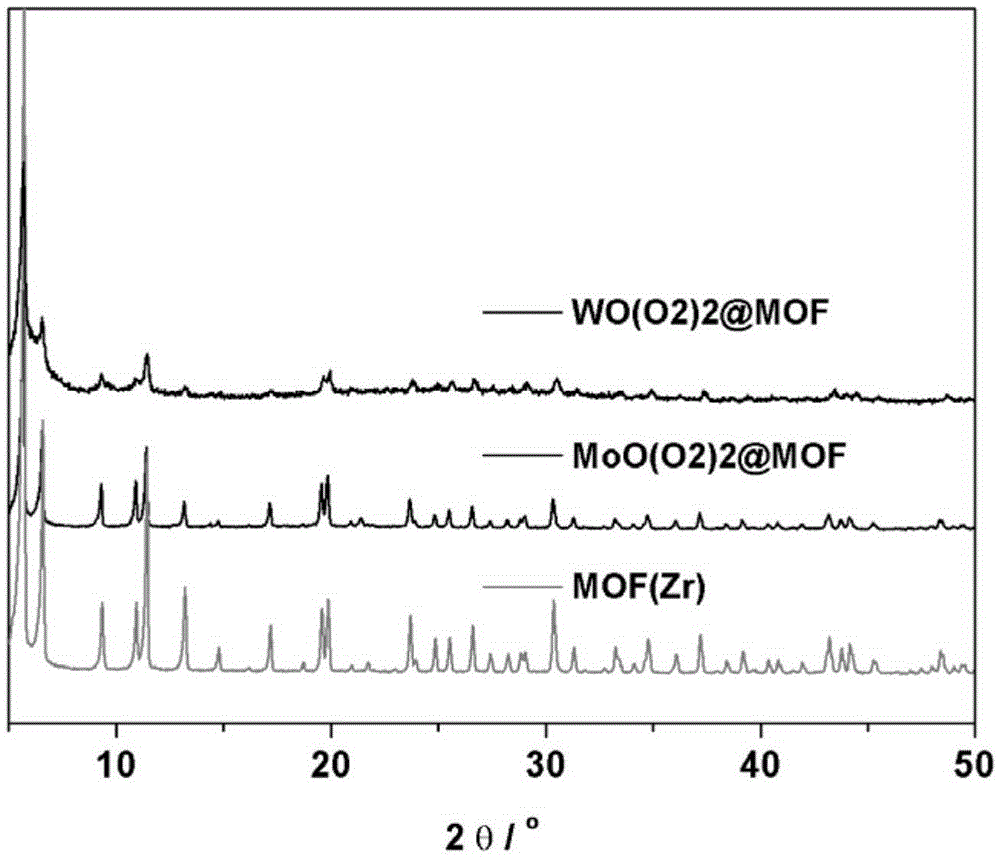
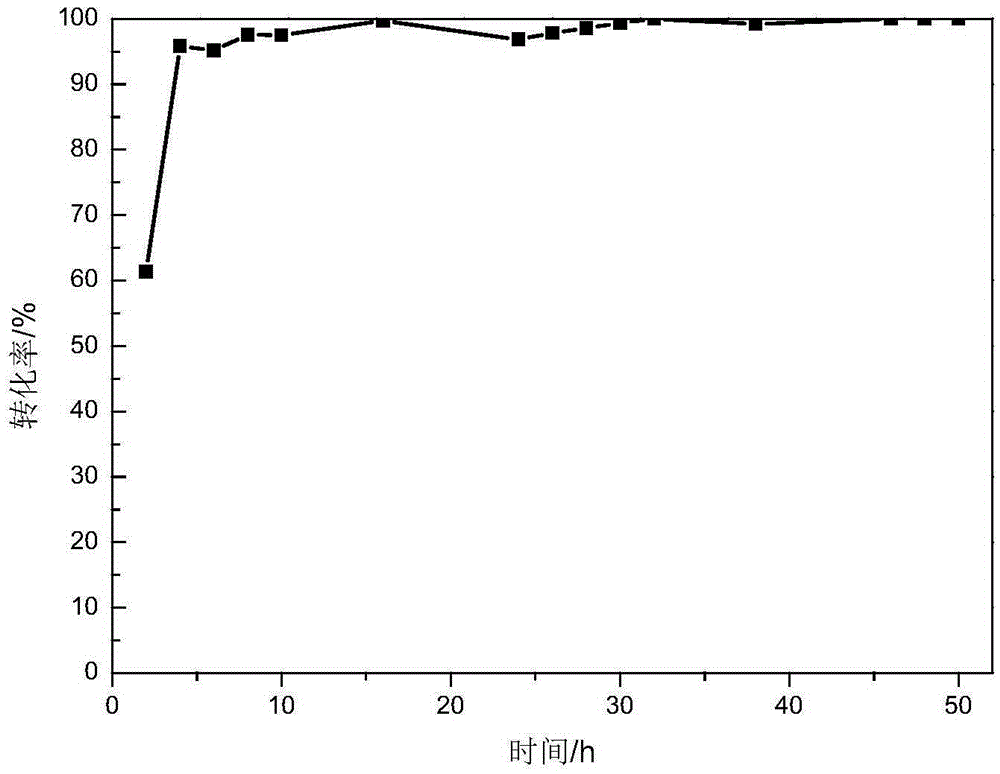
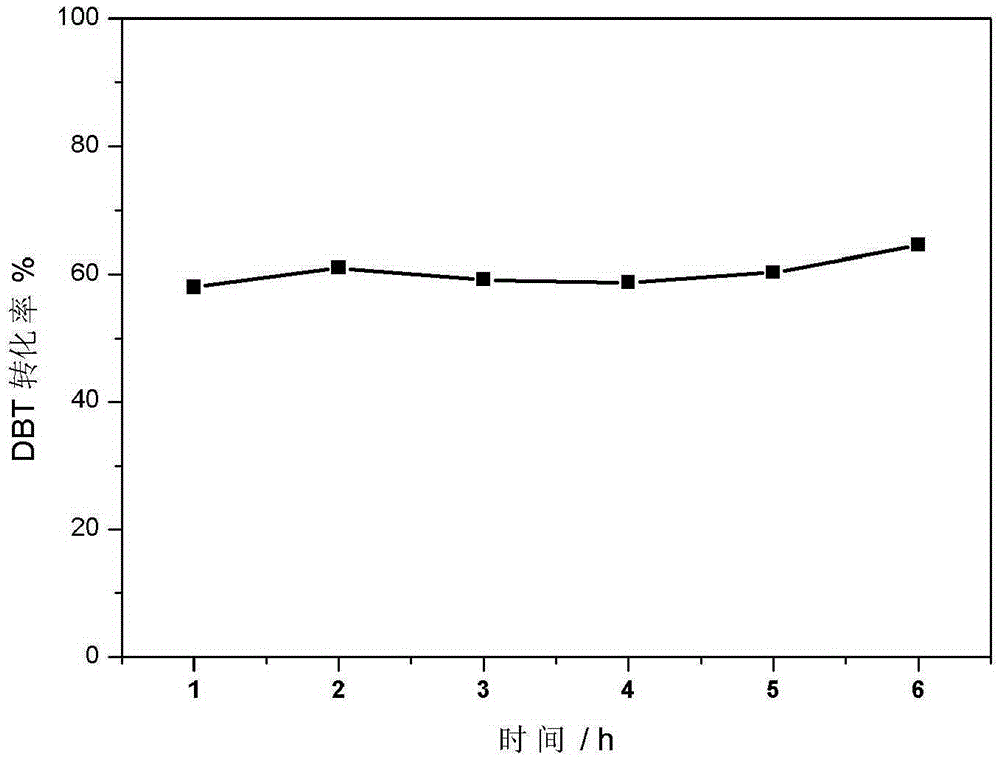
MOF supported metal peroxide catalyst
A metal peroxide, metal peroxide technology, applied in physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, refining with oxygen-containing compounds, etc., can solve the problem of catalyst deactivation, active group The problem of separation loss, etc., to achieve the effect of high activity, good stability, and stable catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] About the preparation method of above-mentioned catalyst, more specific embodiment is as follows:
[0044] In MO(O 2 ) 2 , (M=Mo 6+ , W 6+ ) instead of the commonly used MO 3 (M=Mo 6+ , W 6+ ) as the main active component of the catalyst. The specific preparation steps are: weigh 2-50g MoO 3 And 15ml~500ml30% H 2 o 2Transfer the aqueous solution into a 50-1000ml round-bottom flask, stir overnight at room temperature to 90°C, filter and separate, add a certain amount of DMF solvent to the collected filtrate, refrigerate and crystallize in the refrigerator for 1-14 days, and then carry out vacuum filtration until dry. Get MoO(O 2 ) 2 2DMF. The content of the active components of the catalyst is between 5% and 50% in terms of mass fraction (calculated on the basis of the total mass of the catalyst).
[0045] Select an organic ligand containing a bipyridyl chelate coordination site to coordinate with a zirconium salt to prepare a zirconium-based metal-organic f...
Embodiment 1
[0053] Embodiment 1, the preparation of metal organic framework material
[0054] Preparation of MOF(Zr): Weigh 0.13g 2,2’-bipyridine-5,5’-dicarboxylic acid, 0.38g biphenyl dicarboxylic acid, 0.49g ZrCl 4 , dissolved in 100ml of N,N'-dimethylformamide (DMF), followed by the addition of 3.6ml of acetic acid. Reactions were carried out in 150ml round bottom flasks. After stirring at room temperature for 30 minutes, the temperature was raised to 120° C., and stirred at constant temperature for 36 hours. After suction filtration and drying, the solid was transferred to the above-mentioned round-bottomed flask, and 100 ml of DMF was added and stirred at a constant temperature in an oil bath at 80° C. for 3 h. After suction filtration and drying, the resulting solid was transferred to a Soxhlet extractor and extracted with ethanol overnight. The solid powder obtained after drying is MOF(Zr).
Embodiment 2
[0055] Embodiment 2, the preparation of metal peroxide
[0056] MoO(O 2 ) 2 Preparation: weigh 10gMoO 3 and 50ml30% H 2 o 2 The aqueous solution was transferred into a 100ml round bottom flask, stirred overnight at 45°C, filtered and separated, a certain amount of DMF solvent was added to the collected filtrate, refrigerated and crystallized in the refrigerator for three days, and then vacuum filtered to dryness to obtain MoO(O 2 ) 2 • 2DMF (8.9 g, 0.0276 mol).
[0057] WO(O 2 ) 2 Preparation of hydrogen peroxide solution: weigh tungstic acid (9.3g), measure H 2 o 2 (80ml) in the round-bottomed flask of 100ml, stirred overnight at room temperature, centrifuged then, the liquid product of collecting is WO(O 2 ) 2 of hydrogen peroxide solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com