Eugenol synthesis method
A synthesis method and technology for eugenol, applied in the field of eugenol synthesis, can solve problems such as large influence, complex production process, unstable source, etc., and achieve the effects of saving production cost, shortening process time, and being easy for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The synthetic method of catalyst THLD comprises the following steps:
[0044] 1. The synthesis of catalyst THLD: add 2L concentration to be 0.5mol / LCu(NO 3 ) 2 and 0.1mol / LCo(NO 3 ) 2 The mixed solution was stirred evenly and heated to 75°C;
[0045] 2. After cooling down, add 240g of urea, transfer to a high-pressure reactor, and conduct a hydrothermal reaction at 200°C for 12 hours.
[0046] 3. Suction filter after cooling, dry the filter residue and put it into a horse boiling furnace for calcination, set the calcination temperature to 900°C, add 3
[0047] Double the weight ratio of tartaric acid + EDTA,
[0048] 4. Adjust the pH to 11 with 28% ammonia water to obtain the product.
[0049] 5. Obtain about 750 g of catalyst.
Embodiment 2
[0051] A kind of eugenol synthetic method, concrete steps are as follows:
[0052] 1) Add 20ml tap water in the reactor, then add under the state of stirring, 3.38g sodium hydroxide, 10g guaiacol, 6.48g chloropropene, 16g sodium chloride and 0.3g catalyst THLD, control the reaction temperature at 11.51 (g) crude eugenol was obtained after reaction at 20°C for 1 hour.
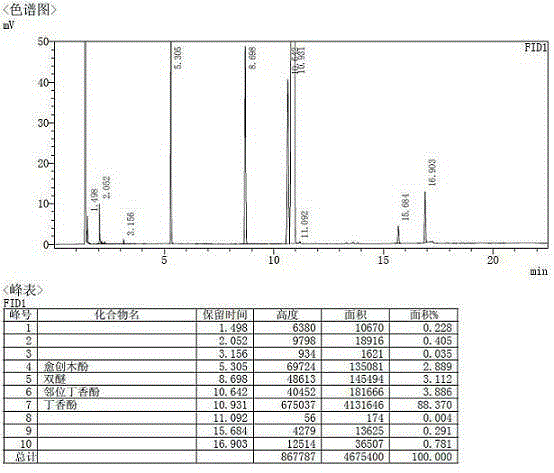
[0053] The conversion rate of guaiacol reaches 98%, and the yield of eugenol reaches 88%; figure 1 As shown, the GC purity of the product is 88.37%.
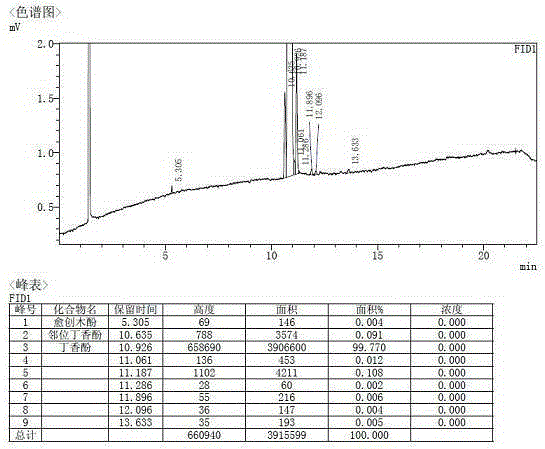
[0054] The product was rectified to separate guaiacol and bisethers. The remaining material was ortho eugenol and eugenol. Add 3 g of potassium carbonate and 15 ml of n-octane to the remaining material, stir at room temperature for 6 hours and then filter. The filter cake was placed in hot water Dissolve in medium, separate layers after dissolving, and obtain eugenol product through simple distillation of the oil phase. Such as figure 2 As shown, the GC purit...
Embodiment 3
[0056] A kind of eugenol synthetic method is characterized in that: concrete steps are as follows:
[0057] 1) Preparation of sodium phenolate: put 20ml of tap water into the preparation kettle, start stirring, then put in 3.22g of sodium hydroxide and 10g of guaiacol, then stir and cool down to 16°C, and finally transfer to the wood phenoxide storage tank;
[0058] 2) Put 12ml of water into the reactor, start stirring, and put in 19 sodium chloride and 0.3g catalyst THLD in turn;
[0059] 3) Cool down to 12°C;
[0060] 4) Add 6.78g of allyl chloride, and then start to add dropwise the sodium phenate in the wood phenate storage tank, dropwise at a constant speed, and dropwise for 2 hours;
[0061] 5) Control the reaction temperature to 22°C during the dropwise addition;
[0062] 6) After the dropwise addition, the reaction temperature was controlled at 27° C.; the reaction time was 1 hour to obtain 12.23 g of crude eugenol.
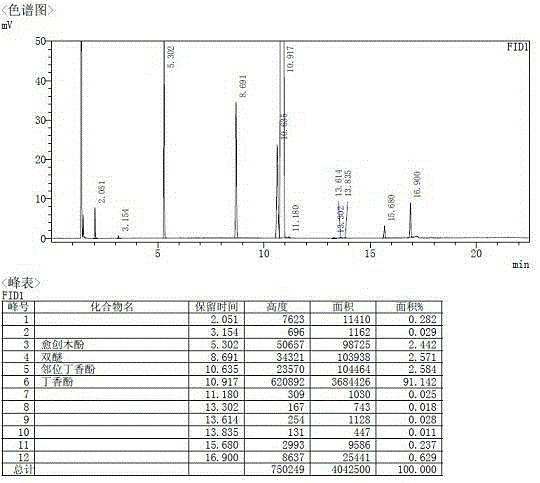
[0063] The conversion rate of guaiacol reaches 98%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com