A kind of determination method of total phospholipid content in hemoglobin oxygen-carrying medicine
A technology of total phospholipid content and determination method, which is applied in the field of determination of total phospholipid content in hemoglobin oxygen-carrying drugs, can solve the problem of inability to quickly detect the total phospholipid content of hemoglobin oxygen-carrying drugs, complicated operations, and detection sensitivity that cannot meet the requirements, etc. problems, achieve good economic effects, improve detection throughput, and shorten sample detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation of malachite green solution: Precisely weigh 0.4 mg of malachite green, dissolve it in 100 ml of Pall ultrapure water, and prepare a malachite green solution with a weight content of 0.4%.
[0050] Preparation of ammonium molybdate solution: Accurately weigh 1.008 g of ammonium molybdate, dissolve it with 5 mol / L hydrochloric acid, and prepare ammonium molybdate solution with a weight content of 4.2%.
[0051] Preparation of Tween 20 solution: Accurately weigh 0.375 g of Tween 20, dissolve it in 25 ml of Pall ultrapure water, and prepare a Tween 20 solution with a weight content of 1.5%.
[0052] Preparation of the storage solution: the malachite green solution and the ammonium molybdate solution were mixed at a volume ratio of 3:1, filtered, and stored at room temperature in the dark for 2 to 3 weeks to obtain a storage solution.
[0053] Preparation of inorganic phosphorus chromogenic solution: mix the storage solution with the Tween 20 solution at a volum...
Embodiment 2
[0059] Sample to extract volume ratio selection test: At ordinary room temperature, when the sample to extract volume ratio is 1:4 and 1:8, it is easy to cause rapid protein denaturation of the hemoglobin solution sample, which may affect the extraction effect. Therefore, the prepared extract was pre-frozen in a deep-low temperature refrigerator for 30 minutes in advance.
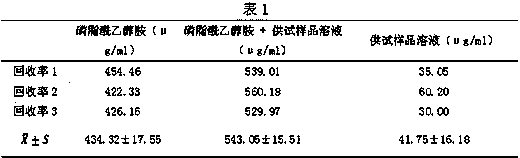
[0060] When the volume ratio of sample to extract is 1:1, 1:2, 1:4, 1:8, in experiments with volume ratio of 1:1 and 1:2, the hemoglobin solution turns pink as the temperature returns to room temperature , the hemoglobin did not undergo severe polymerization and denaturation; while in experiments with volume ratios of 1:4 and 1:8, as the temperature returned to room temperature, the hemoglobin solution turned dark red, indicating that the hemoglobin was polymerized and denatured. The centrifugation operation was performed at room temperature, the speed of the centrifuge was 5000 rpm, and the centrifugation ...
Embodiment 3
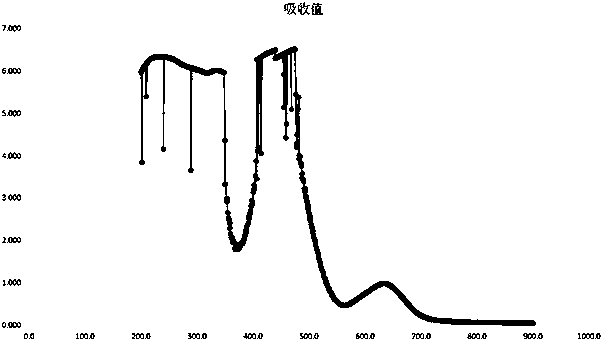
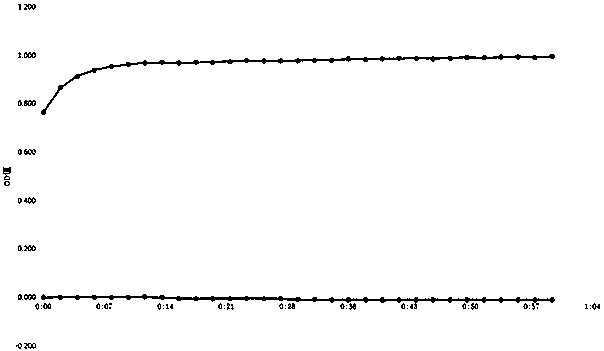
[0062] Full-wavelength scanning and reaction kinetic curve of potassium dihydrogen phosphate standard solution: Measure 300 μl of potassium dihydrogen phosphate standard solution in a quartz cuvette, then add 100 μl of high-grade pure perchloric acid and 2ml of inorganic phosphorus chromogenic solution, and use Ultrospec6300pro UV The visible spectrophotometer scans the full wavelength from 200-900nm, the scanning wavelength interval is 1nm, and the scanning speed is 2649nm / min; from the beginning of adding the inorganic phosphorus chromogenic solution, a full-wavelength scanning is performed every 5 minutes, and the recording time is 0min, 5min, 10min, 15min, 20min. Measurement results such as figure 1 As shown, the reaction measurement wavelength can be used for determination at 560-690nm wavelength, and the maximum absorption peak corresponds to 634nm wavelength, so when using Ultrospec 6300pro ultraviolet-visible spectrophotometer to detect the test sample solution and pot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com