High performance liquid chromatography method for analysis of alkyl chloride compound
A high-performance liquid chromatography and alkyl acid chloride technology, which is applied in the field of high-performance liquid chromatography to analyze alkyl acid chloride compounds, can solve problems such as inability to separate R and S configurations, strong pungent odor, and needs to be improved. Sensitivity, convenient liquid phase detection, good elution and separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
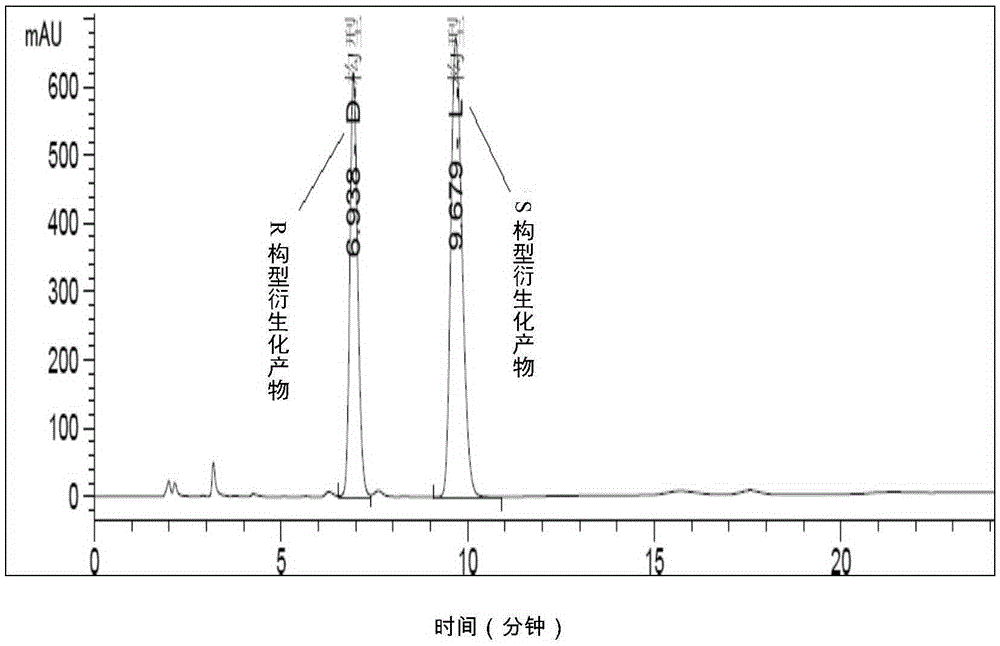
[0041] Embodiment 1 adopts high performance liquid chromatography to measure the content of S-acetoxy propionyl chloride enantiomer
[0042] Instrument: Agilent1100 HPLC, 1100 UV detector
[0043] Chromatographic column: CHIRALPAKAS-H (150×4.6mm, 5μm);
[0044] Mobile phase: n-hexane: isopropanol = 85: 15 (v / v)
[0045] Flow rate: 1.0mL / min
[0046] Detection wavelength: 210nm
[0047] Column temperature: 35°C
[0048] Injection volume: 2μL
[0049] Diluent: n-Hexane: Isopropanol = 50:50 (v / v)
[0050] Run time: 25 minutes
[0051] Experimental steps:
[0052] Derivatization method: Dissolve about 80 mg of benzylamine in dichloromethane, then slowly add about 60 mg of S-acetoxypropionyl chloride sample under ice bath, stir for 10 minutes after the addition, filter the solid, and wash with a small amount of dichloromethane , the collected solid is the derivatized sample of S-acetoxypropionyl chloride, and is set aside. (Note: The product obtained by derivatization of S-c...
Embodiment 2
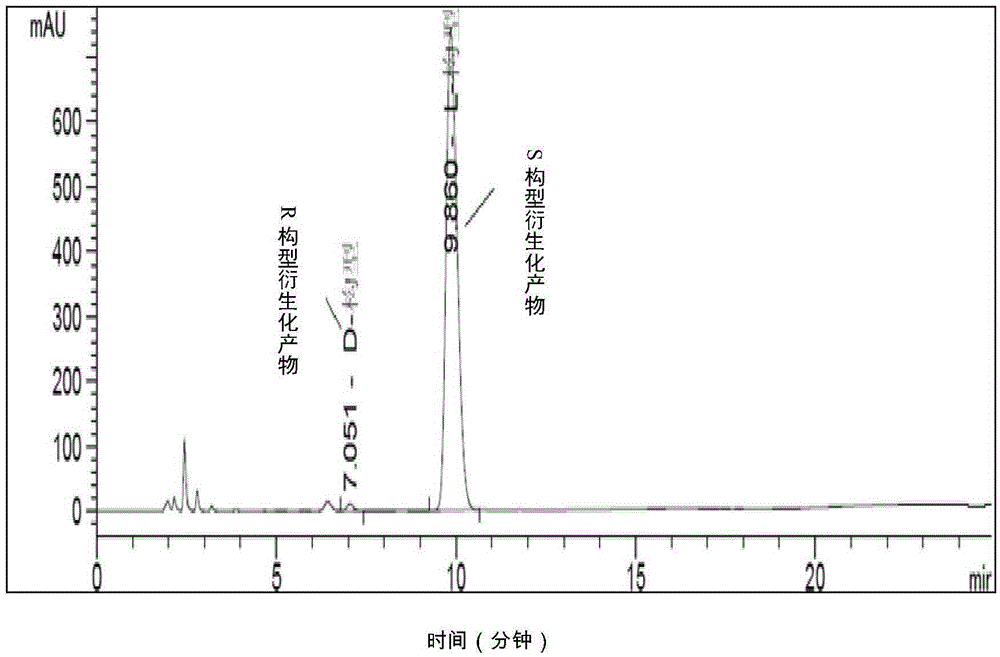
[0086] Example 2 Determination of the content of S-acetoxybutyryl chloride enantiomers by high performance liquid chromatography
[0087] Instrument: Agilent1100 HPLC, 1100 UV detector
[0088] Chromatographic column: CHIRALPAKAS-H (150×4.6mm, 5μm);
[0089] Mobile phase: n-hexane: isopropanol = 85: 15 (v / v)
[0090] Flow rate: 1.0mL / min
[0091] Detection wavelength: 210nm
[0092] Column temperature: 35°C
[0093] Injection volume: 2μL
[0094] Diluent: n-Hexane: Isopropanol = 50:50 (v / v)
[0095] Run time: 25 minutes
[0096] Experimental steps:
[0097] Derivation step: Dissolve about 40 mg of benzylamine in dichloromethane, then slowly add about 32 mg of acid chloride under ice bath, stir for 10 minutes after the addition, filter the solid, wash with a small amount of dichloromethane, and collect the solid as acetoxy The derivatized sample of butyryl chloride is reserved.
[0098] Sample preparation: take about 20mg of the derivatized sample and place it in a 5ml...
Embodiment 3
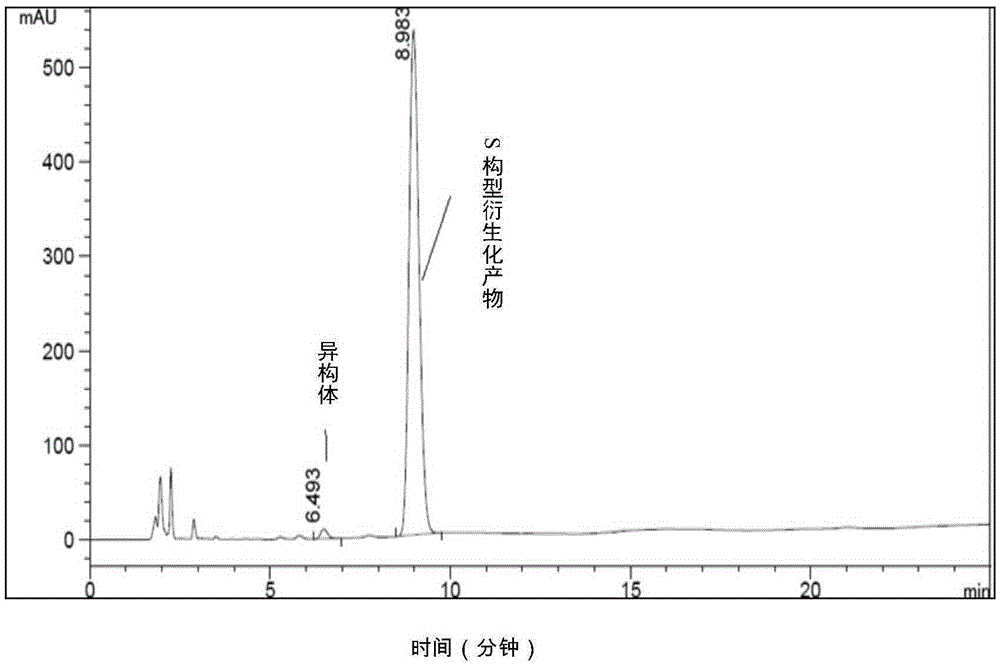
[0100] Example 3 Determination of the content of S-acetoxypropionyl chloride enantiomers by high performance liquid chromatography
[0101] Instrument: Agilent1100 HPLC, 1100 UV detector
[0102] Chromatographic column: CHIRALPAKAD-H (250×4.6mm, 5μm);
[0103] Mobile phase A: n-hexane: ethanol = 90: 10 (v / v)
[0104] Flow rate: 1mL / min
[0105] Detection wavelength: 210nm
[0106] Column temperature: 35°C
[0107] Injection volume: 2μL
[0108] Diluent: n-Hexane: Ethanol = 50:50 (v / v)
[0109] Run time: 25 minutes
[0110] Derivatization step: Dissolve about 40 mg of benzylamine in dichloromethane, then slowly add about 30 mg of acid chloride under ice bath, stir for 10 minutes after the addition, filter the solid, wash with a small amount of dichloromethane, collect the solid as acetoxy The derivatized sample of propionyl chloride is used for later use.
[0111] Sample preparation: Take about 20mg of the derivatized sample in a 5ml volumetric flask, dissolve it with a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com