Cathode material used for lithium sulfur battery, preparation and application thereof
A cathode material, lithium-sulfur battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of sulfur electrochemical inertness and poor cycle stability, reduced battery charge and discharge efficiency, and low electrode capacity utilization, etc. The effect of cycle stability, good selectivity, good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
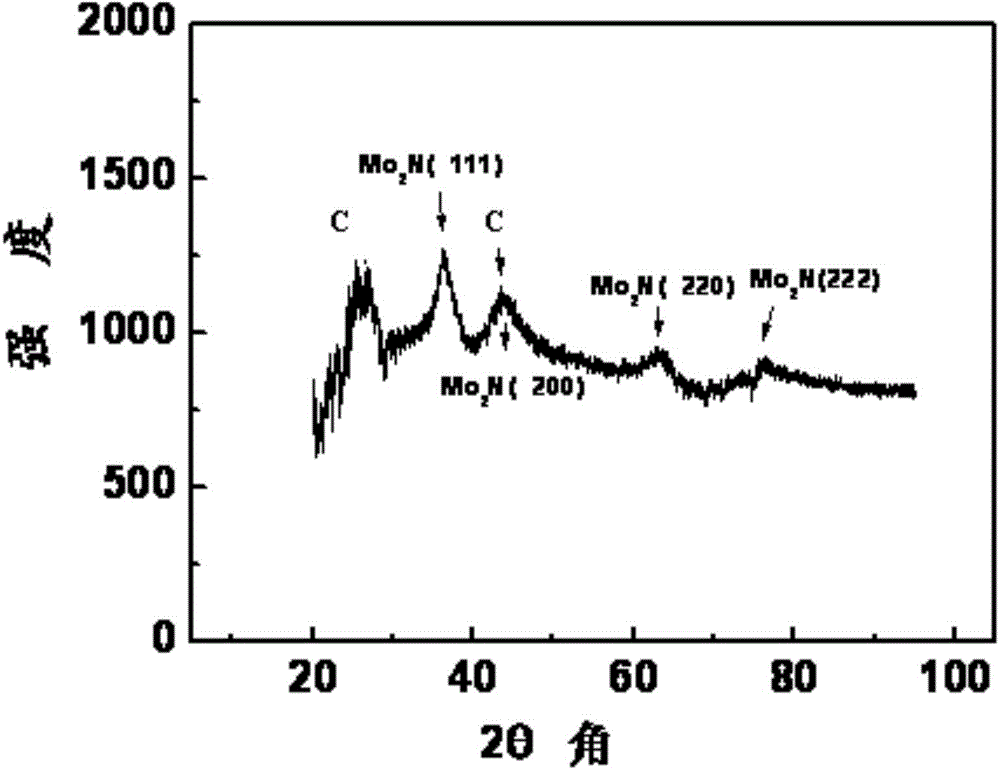
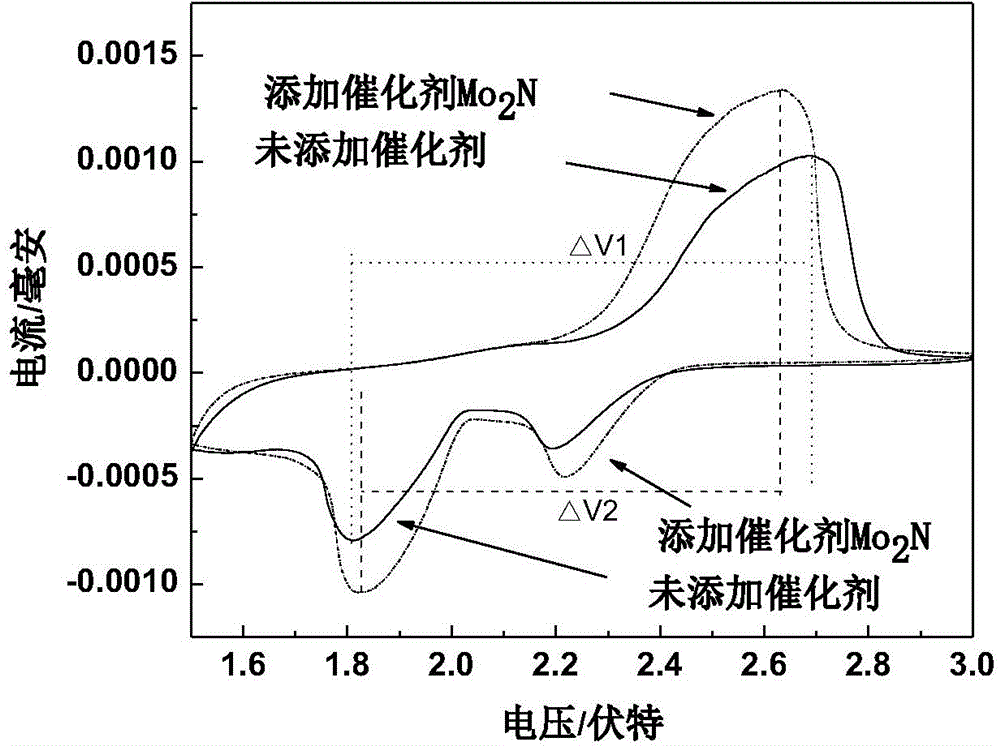
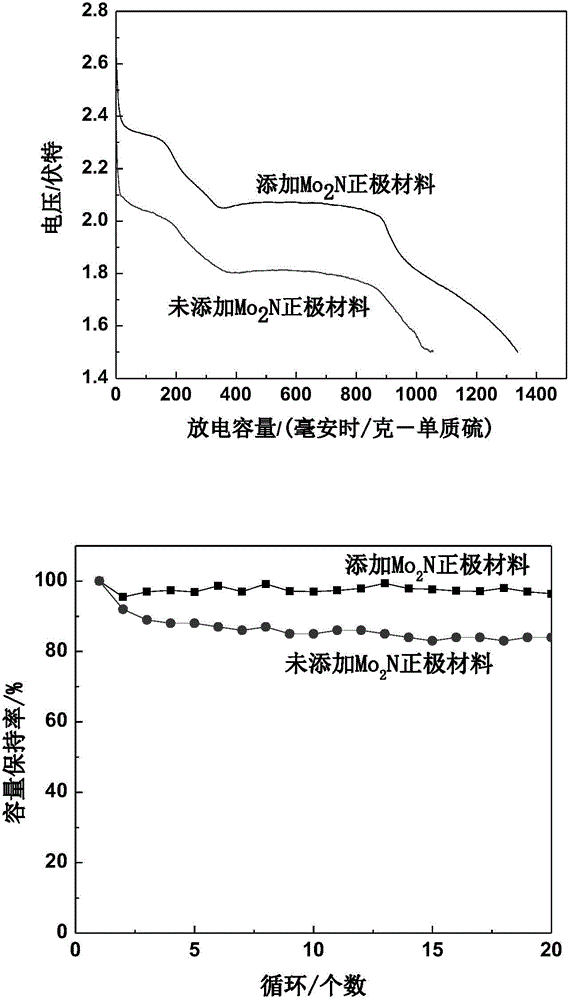
[0039] Take 0.83ml of ammonium molybdate aqueous solution with a concentration of 133.6mgMo / ml, add appropriate amount of water and isopropanol and ultrasonically mix it evenly (the mass percentage of ammonium molybdate is 5%), add conductive carbon Ketjen black, Ketjen black The mass percentage is 50%, stirred for half an hour to mix evenly, and soaked for 12 hours. The above mixture was slowly evaporated on a water bath at 90°C until the surface was dry, and then dried in a vacuum oven at 120°C for 2 hours; then the sample was moved into a tube furnace for sintering and nitriding. The roasting condition is N 2 In the atmosphere, the temperature was raised to 500°C at a constant temperature of 5°C / min for 2 hours, and then lowered to room temperature. Then pass 60ml / min of NH 3 Raise the temperature from room temperature to 623K at 5K / min; then raise the temperature from 623K to 723K at a speed of 0.5K / min, then raise the temperature from 723K to 973K at 2.5K / min, keep the ...
Embodiment 2
[0044] Take 0.25ml of ammonium metatungstate aqueous solution with a concentration of 441.216mgW / ml, add appropriate amount of water and isopropanol and ultrasonically mix it evenly (ammonium metatungstate mass percentage is 60%), add carbon nanotubes, carbon nanotubes The mass percentage is 10%, stirred for half an hour to mix evenly, and soaked for 12 hours. The above mixture was slowly evaporated on a water bath at 90°C until the surface was dry, and then dried in a vacuum oven at 120°C for 12 hours; then the sample was moved into a tube furnace for sintering and nitriding. The roasting condition is N 2 In the atmosphere, the temperature was raised to 500°C at a constant temperature of 5°C / min for 2 hours, and then lowered to room temperature. Then pass 60ml / min of NH 3 Raise the temperature from room temperature to 623K at 5K / min; then raise the temperature from 623K to 723K at a speed of 0.5K / min, then raise the temperature from 723K to 1025K at 2.5K / min, keep the tempe...
Embodiment 3
[0046] The precursor oxide of supported cobalt molybdenum nitride is impregnated: 0.59ml of Co(NO with a concentration of 134.4mgCo / ml 3 ) 2 ·6H 2 O aqueous solution was slowly added to 0.96ml of ammonium molybdate aqueous solution with a concentration of 133.6mgMo / ml, and the mixed solution was immersed in 0.5g of carbon nanofibers, stirred for half an hour to mix evenly, and then immersed for 12 hours. The above mixture was slowly evaporated on a water bath at 90°C until the surface was dry, and then dried in a vacuum oven at 120°C for 12 hours; then the sample was moved into a tube furnace for sintering and nitriding. The roasting condition is N 2 In the atmosphere, the temperature was raised to 500°C at a constant temperature of 5°C / min for 2 hours, and then lowered to room temperature. Then pass 60ml / min of NH 3 Raise the temperature from room temperature to 623K at 10K / min; then raise the temperature from 623K to 723K at a speed of 0.5K / min, then raise the temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com