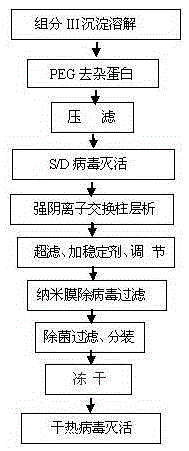
Method for preparing human prothrombin complex from Cohn blood plasma component III
A technology of human prothrombin and complexes, which is applied in the field of preparation of human prothrombin complexes, can solve the problems of unpredictable effects of protein separation and residual gelatin, reduce the probability of virus contamination, and prevent the activation of thrombin , no cross-contamination effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
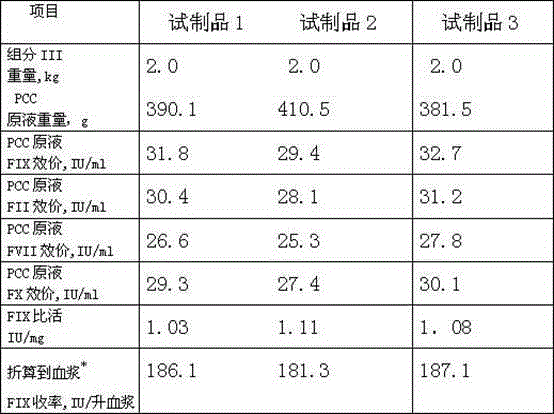
Embodiment 1
[0042] 1, Component III dissolution: suspend 2kg of component III precipitate in 10kg dissolution buffer, the dissolution buffer formula is 0.02M sodium citrate, 0.15M sodium chloride, pH6.50-6.60; stir for 4 hours to fully dissolve the precipitate ;
[0043] 2, PEG precipitation to remove impurities: add 50% PEG solution to the above suspension until the PEG content in the suspension is 3%, press filter after stirring for 1.5 hours, the filter plate is Supradur50P+1.0μm filter element of PALL company, and the filter plate is pre-used in the steps ( 1) Pre-washing with the dissolution buffer, and collecting a clear filtrate;
[0044] 3. S / D virus inactivation: Add Tween80 to 1.0% (wt%) and TNBP (tributyl phosphate) to 0.3% (wt%) to the above filtrate, stir well, heat up to 24-26°C, and keep warm for 6 Hour;
[0045] 4. Strong anion exchange column chromatography: Cool the above S / D solution to 8°C, and then put it on the Capto-Q column. The column is pre-filled with equilibr...
Embodiment 2
[0053] 1, Dissolution of Component III: Suspend 2kg of Component III precipitate in 20kg of dissolution buffer, the formulation of the dissolution buffer is 0.02M sodium citrate, 0.15M sodium chloride, pH7.40-7.50; stir for 2 hours to fully dissolve the precipitate ;
[0054] 2. PEG precipitation to remove impurities: add 50% PEG solution to the above suspension until the PEG content in the suspension is 7%, press filter after stirring for 0.5 hours, the filter plate is Supradur50P+1.0μm filter element of PALL company, and the filter plate is used in advance 1, the dissolution buffer pre-washed, collected to obtain clarified filtrate;
[0055] 3, S / D virus inactivation: with embodiment one;
[0056] 4. Strong anion exchange column chromatography: cool down the above S / D solution to 20°C, and then put it on the QSepharoseFF column. Sodium,) balance; after loading the column, wash the column with the above equilibration buffer, and then wash the column with the elution buffer...
Embodiment 3
[0063] 1, Dissolution of component III: Suspend 2kg of component III precipitate in 20kg of dissolution buffer, the composition of the dissolution buffer is 0.02M sodium citrate, 0.15M sodium chloride, pH6.90-7.10; stir for 3 hours to fully dissolve the precipitate ;
[0064] 2. PEG precipitation to remove impurities: Add 50% PEG solution to the above suspension until the PEG content in the suspension is 5%, press filter after stirring for 1 hour, the filter plate is Supradur50P+1.0μm filter element of PALL company, the filter plate is pre-used The dissolving buffer described in step 1 is pre-washed, and the clarified filtrate is collected;
[0065] 3, S / D virus inactivation: with embodiment one;
[0066] 4. Strong anion exchange column chromatography: cool the above S / D solution to 15°C, and then put it on the Capto-Q column, which is pre-filled with equilibrium buffer (PH6. Sodium chloride,) balance; after loading the column, wash the column with the above equilibration b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com