Construction method of stable cell line realizing FTH1 gene controlled expression
A construction method and cell line technology, applied in the field of cell line construction, can solve problems such as cell damage, iron metabolism disorder, and reduced cell proliferation activity, and achieve the effect of precise control and controllable expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A method for preparing a stable cell line with controlled expression of FTH1 gene, comprising the following steps:
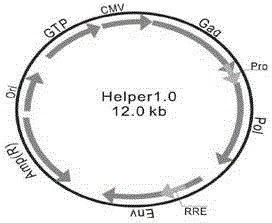
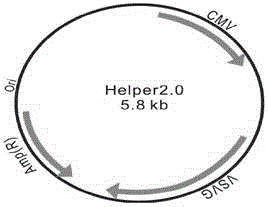
[0056] (1) Construction of viral vector:
[0057] ◆Acquisition of the target gene FTH1: Synthesize the cDNA sequence of the FTH1 gene according to the information of FTH1 (BC000857) in Genebank, design primers, and the upstream and downstream primers have AgeI and EcoRI restriction sites respectively;
[0058] FTH1-F:
[0059] 5'-AACCGTCAGATCGC ACCGGT GCCACCATGACGACCGCGTCCACCTC-3'
[0060] FTH1-R:
[0061] 5'-TCCTTGTAGTCCAT GAATTC GCTTTCATTATCACTGTCTC-3'
[0062] PCR reaction system:
[0063]
[0064] PCR amplification conditions: 94°C, 5min; 94°C, 30s; 55°C, 30s; 72°C, 2min; 30cycle; 72°C, 10min; 4°C storage;
[0065] ◆FTH1 gene product digestion: use AgeI and EcoRI for double digestion;
[0066] Enzyme digestion reaction system:
[0067]
[0068] 37 ℃, reaction 4h; 70 ℃ fire extinguishing 10min;
[0069] ◆Agarose gel electrophoresis of ...
Embodiment 2
[0145] Detection of the SK-N-SH / Tet-on / FTH1 stable cell line constructed in Example 1
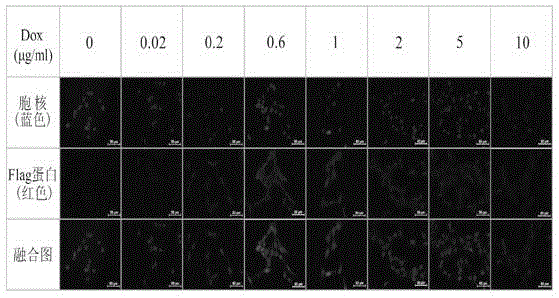
[0146] 1. Western blot assay for the induced expression of FTH1 protein in SK-N-SH cells
[0147] ◆Doxycycline (Dox) induces the expression of FTH1 in cells
[0148] 1) Divide SK-N-SH / Tet-on / FTH1 cells into 8×10 5 Density inoculation into 75ml culture flask, after the cells adhered to the wall, discard the original medium and divide into 2 groups;
[0149] 2) Group 1 needs 8 75ml culture flasks, add complete medium containing doxycycline at different concentrations (0, 0.02, 0.2, 0.6, 1, 2, 5, 10 μg / ml) respectively, and extract the whole cells after culturing for 72 hours. protein;
[0150] 3) The second group needs six 75ml culture flasks, add complete medium containing 0.6μg / ml doxycycline, and extract the whole protein of the cells at 0, 24, 48, 72, 96, and 120 hours respectively;
[0151] ◆Preparation of cell protein samples
[0152] 1) Pour off the original medium in the culture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com