Method for producing chromium oxide green through sodium circulation
A technology of chromium oxide green and chromium oxide, applied in the directions of chromium trioxide, chromium oxide/hydrate, etc., can solve the problems of long production process, high cost and high energy consumption, and achieve short production process, low production cost, The effect of simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
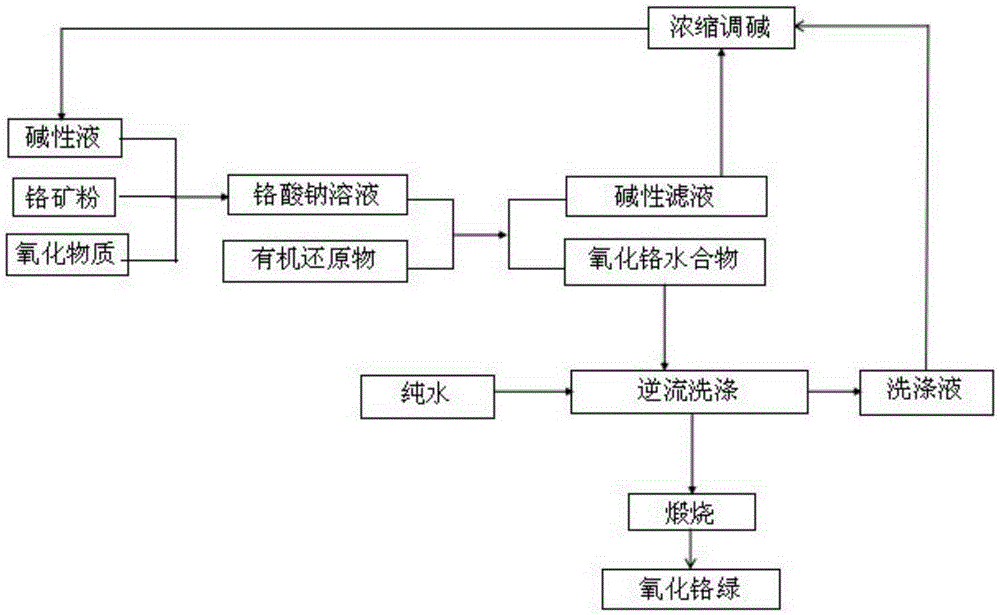
[0028] Step 1: Grind chromite to obtain chromite powder with a particle size of less than 80um;
[0029] Step two, 2.50t chromite powder, 3.50t sodium hydroxide and 5.85m 3 Water is added to the oxidation reaction kettle, oxygen is introduced, and the reaction is heated to 300°C for 3 hours. After the reaction is completed, the temperature is cooled and separated to obtain an alkaline solution of sodium chromate;
[0030] Step 3. Add 1.35t of starch to the alkaline solution of sodium chromate, heat it to 193℃ in the reduction kettle, keep the temperature for 0.6 hours, cool to 20℃, and separate by filtration to obtain chromium oxide hydrate and 4.71m 3 Alkaline filtrate, the alkaline filtrate is Na + Ion concentration 16.56mol / L;
[0031] Step 4. Wash the chromium oxide hydrate countercurrently for 5 times, and recover the washing liquid; then calcinate the washed chromium oxide hydrate in a high-temperature furnace at 1000℃ for 2 hours to obtain 0.96t of chromium oxide green; detect ...
Embodiment 2
[0034] Step 1: Grind chromite to obtain chromite powder with a particle size of less than 80um;
[0035] Step 2. Mix the alkaline filtrate and washing liquid in Example 1 and adjust the alkali as alkaline solution, take 2.40t chromite powder, 3.65m 3 Alkaline solution (Na + Ion concentration 23.63mol / L) was added to the oxidation reactor, oxygen was introduced, and the reaction was heated to 275°C for 1 hour. After the reaction was completed, the temperature was lowered and separated to obtain an alkaline solution of sodium chromate;
[0036] Step 3. Add 1.28t of starch to the alkaline solution of sodium chromate, heat it to 152℃ in the reduction kettle, keep the temperature for 1.8 hours, cool to room temperature, and separate by filtration to obtain chromium oxide hydrate and 2.92m 3 Alkaline filtrate, the alkaline filtrate is Na + The ion concentration is 28.56mol / L;
[0037] Step 4. Wash the chromium oxide hydrate countercurrently for 5 times, and recover the washing liquid; then ...
Embodiment 3
[0040] Step 1: Grind the ferrochrome to obtain ferrochrome powder with a particle size of less than 80um;
[0041] Step 2. Mix the alkaline filtrate and washing liquid in Example 2 to adjust the alkaline solution as alkaline solution, take 1.85t ferrochrome powder, 5.32m 3 Alkaline solution (Na + Ion concentration 7.35mol / L) was added to the oxidation reaction vessel, oxygen was introduced, and the reaction was heated to 285°C for 1.3 hours. After the reaction was completed, the temperature was lowered and separated to obtain sodium chromate alkaline solution;
[0042] Step 3. Add 1.86t of starch to the alkaline solution of sodium chromate, heat it to 178℃ in the reduction kettle, keep the temperature for 1.2 hours, cool to room temperature, and separate by filtration to obtain chromium oxide hydrate and 4.78m 3 Alkaline filtrate, the alkaline filtrate is Na + Ion concentration 8.08mol / L;
[0043] Step 4. Wash the chromium oxide hydrate countercurrently for 3 times, and recover the wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com