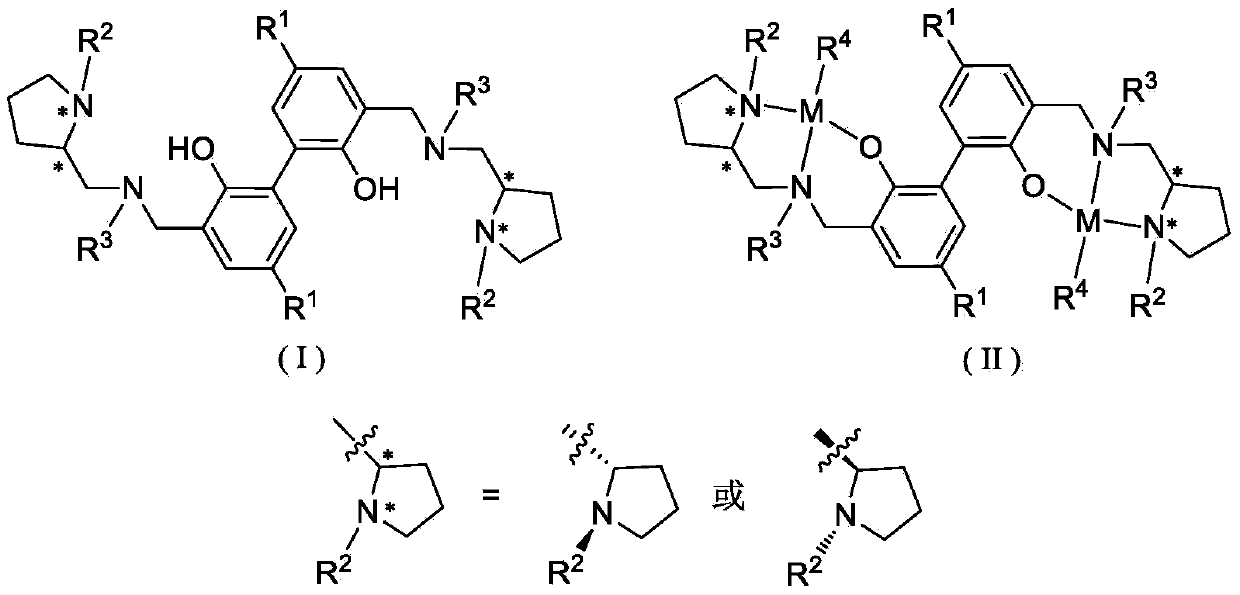
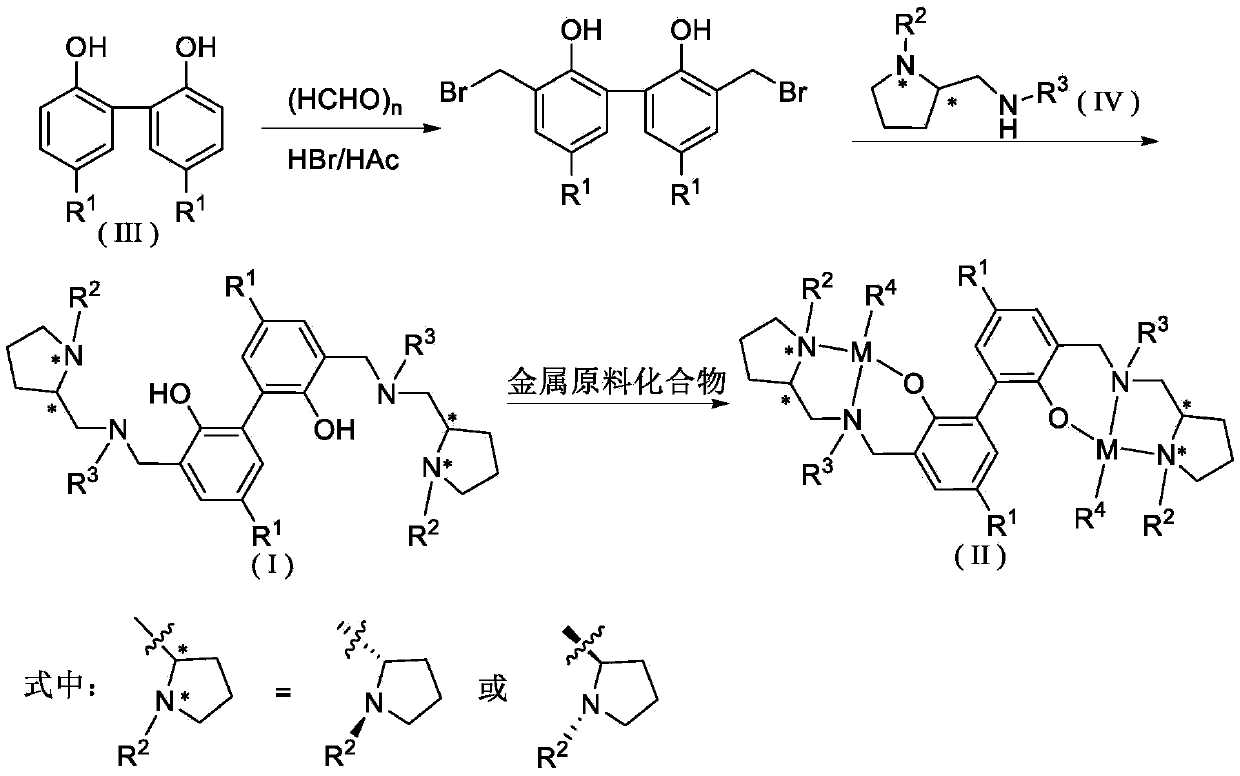
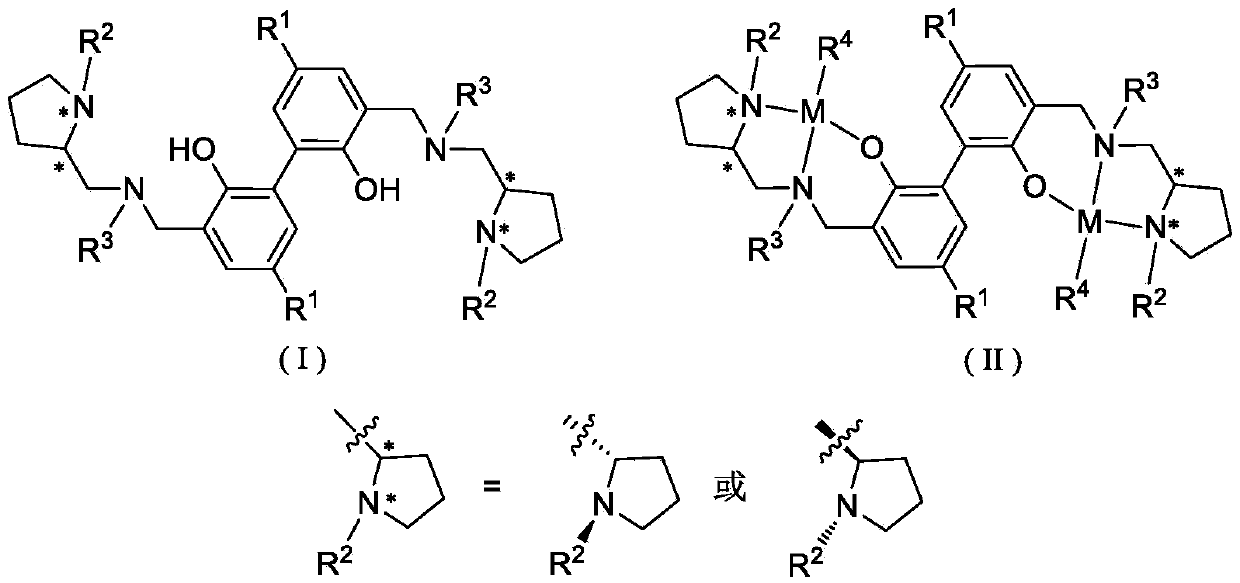
Biphenyl skeleton chiral amino phenol oxyl dual-core zinc-magnesium compound and preparation method and application thereof
A technology of aminophenoloxy binuclear zinc and aminophenol, which is applied in the field of magnesium complexes and biphenyl skeleton chiral aminophenoloxy binuclear zinc, can solve the problem of binuclear zinc and magnesium complexes with few reports and external problems. Racemic lactide has low polymerization activity and no isotactic selectivity, achieving stable properties, high stereoselectivity, and convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of Ligand L1
[0048] (1) Synthesis of 5,5'-dimethyl-3,3'-dibromomethyl-2,2'-biphenol
[0049]
[0050] Add 5,5'-dimethyl-2,2'-biphenol (7.05g, 33.0mmol), paraformaldehyde (3.00g, 100mmol) and 30mL hydrobromic acetic acid solution (30%) into a 100mL three-necked flask , stirred at room temperature for 10 min and then heated to 70 ° C for 2 h. A large amount of white solids precipitated, filtered, washed with petroleum ether, and dried to obtain 8.08 g of white powdery solids, with a yield of 61.2%. 1 HNMR (400MHz, CDCl 3 ): δ7.20(s,2H),7.03(s,2H),5.49(brs,2H),4.60(s,4H),2.31(s,6H).
[0051] (2) Synthesis of Ligand L1
[0052]
[0053] Under the protection of argon, the toluene solution of 5,5'-dimethyl-3,3'-dibromomethyl-2,2'-biphenol (2.80g, 7.00mmol) was slowly dropped into (S)-N -Methylene-[2-(1-ethyltetrahydropyrrolyl)]benzylamine (3.06g, 14.0mmol) and potassium hydroxide (1.57g, 28.0mmol) in toluene solution, heated to 70°C for overnight reacti...
Embodiment 2
[0056] Synthesis of Ligand L2
[0057]
[0058]In addition to raw materials, 5,5'-dimethyl-3,3'-dibromomethyl-2,2'-biphenol (5.00g, 12.5mmol), (S)-N-methylene-[2 -(1-n-butyltetrahydropyrrolyl)] benzylamine (6.16g, 25.0mmol) and potassium hydroxide (2.81g, 50.0mmol), the rest of the operation steps are the same as in Example 1, to obtain a light yellow viscous substance 3.91 g, yield 42.8%.
[0059] 1 HNMR (400MHz, CDCl 3 ):δ7.35-7.28(m,4H),7.28-7.17(m,6H),7.03(s,2H),6.85(s,2H),4.03(d,J=13.6Hz,2H),3.81( d,J=13.0Hz,2H),3.68(d,J=13.7Hz,2H),3.43(d,J=13.0Hz,2H),2.68-2.42(m,8H),2.29(s,6H), 2.10-1.90(m,6H),1.65-1.50(m,4H),1.47-1.33(m,6H),1.33-1.18(m,6H),0.88(t,J=7.2Hz,6H). 13 CNMR (100MHz, CDCl 3 ): δ152.4, 137.1, 131.4, 129.9, 128.9, 128.3, 127.6, 127.3, 126.0, 122.4, 62.4, 59.2, 58.9, 58.6, 55.2, 54.2, 30.9, 30.4, 22.5, 20.8, 20.5, 14Calcd.0.Anal. forC 48 h 66 N 4 o 2 : C, 78.86; H, 9.10; N, 7.66%. Found: C, 78.56; H, 9.22; N, 7.45%.
Embodiment 3
[0061] Synthesis of Ligand L3
[0062] (1) Synthesis of 5,5'-di-tert-butyl-3,3'-dibromomethyl-2,2'-biphenol
[0063]
[0064] Add 5,5'-di-tert-butyl-2,2'-biphenol (14.9g, 50.0mmol), paraformaldehyde (4.50g, 150mmol) and 80mL hydrobromic acetic acid solution (30% ), stirred at room temperature for 10 min and then heated to 70 °C for 2 h. A large amount of white solids precipitated, filtered, washed with petroleum ether, and dried to obtain 17.2 g of white powdery solids, with a yield of 71.2%. 1 HNMR (400MHz, CDCl 3 ): δ7.40(s,2H),7.23(s,2H),5.51(brs,2H),4.65(s,4H),1.28(s,18H).
[0065] (2) Synthesis of Ligand L3
[0066]
[0067] In addition to raw materials, 5,5'-di-tert-butyl-3,3'-dibromomethyl-2,2'-biphenol (7.26g, 15.0mmol), (S)-N-methylene-[ Except for 2-(1-n-butyltetrahydropyrrolyl)]benzylamine (7.39g, 30.0mmol) and potassium hydroxide (3.36g, 60.0mmol), the remaining operating steps were the same as in Example 1 to obtain a light yellow viscous substance 4.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com