Preparation method of castor-oil base branched polybasic cyclic carbonate and non-isocyanate polyurethane thereof
An oil-based non-isocyanate and polyurethane technology, applied in polyurea/polyurethane coatings, organic chemistry, coatings, etc., can solve the problem of reduced reactivity, low number of cyclocarbonates, and poor design and control of cyclocarbonate groups To achieve the effect of high reactivity and high renewable carbon content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
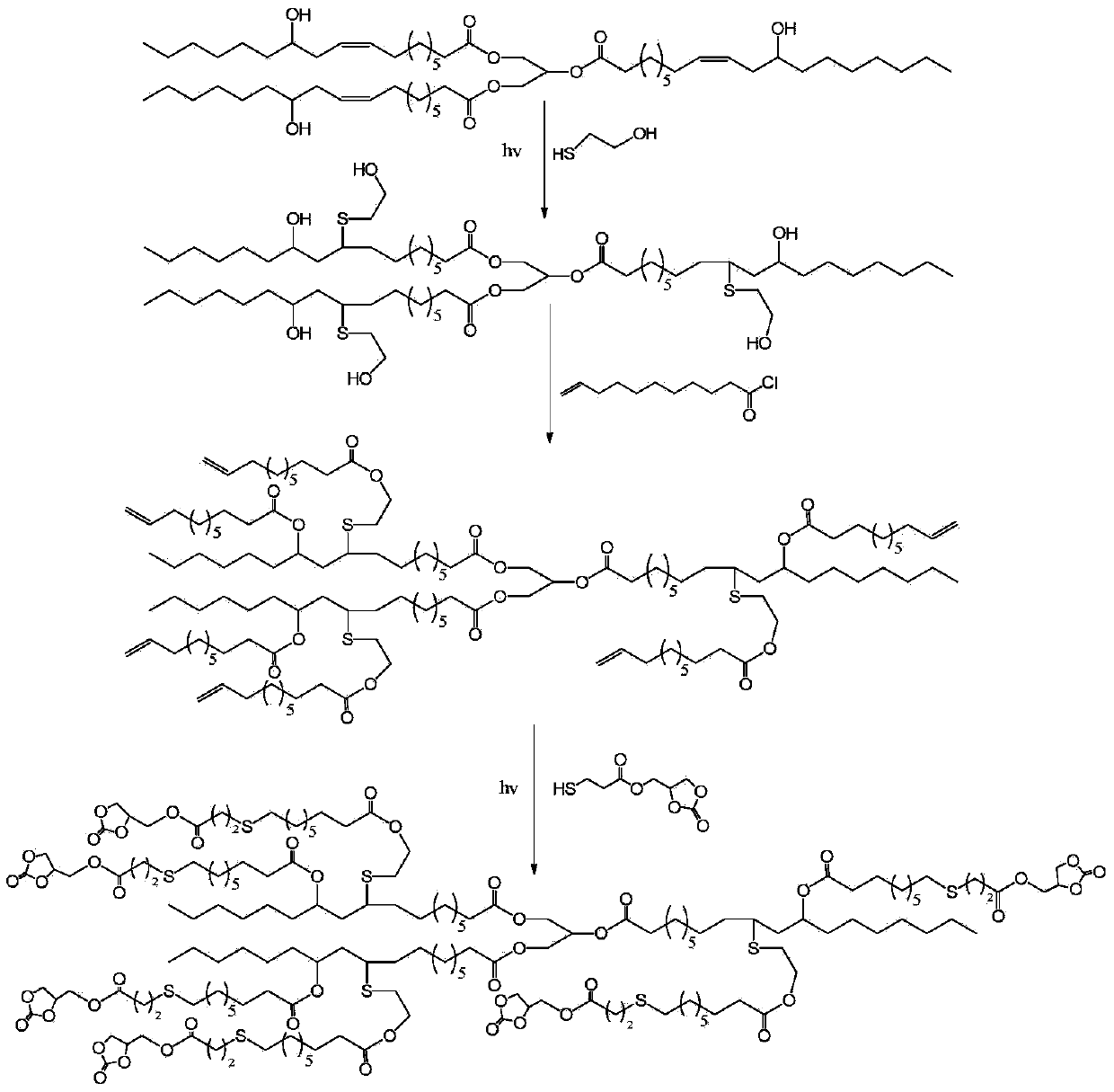
[0020] 1) Preparation of mercapto cyclocarbonate
[0021] Add 11.8g of glycerol cyclocarbonate, 13g of 3-mercaptopropionic acid, 1.24g of p-toluenesulfonic acid, and 40ml of dichloromethane into the reaction flask, and reflux at 80°C for 8h. After the solution was washed three times with deionized water, it was dried with anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation to obtain 16.27 g of mercapto cyclocarbonate with a yield of 79%. Its NMR data are as follows:
[0022] 1 HNMR (400MHz, CDCl 3 , δppm) 4.95 (ddd, J = 9.1, 6.1, 3.8Hz, 1H), 4.58 (t, J = 8.6Hz, 1H), 4.43 (dd, J = 12.6, 3.3Hz, 1H), 4.35 (dd, J =5.2,2.6Hz,1H),4.32(d,J=5.3Hz,1H),2.81(td,J=7.9,4.0Hz,2H),2.74(dd,J=10.2,3.9Hz,2H),1.71 -1.61(m,1H).
[0023] 13 CNMR (100MHz, CDCl 3 ,δppm) 171.18(OC=O), 154.56(OC(O)O), 73.82(CH), 65.19(CH 2 ), 63.24 (CH 2 ), 38.09 (CH 2 ), 19.45 (CH 2 ).
[0024] 2) Preparation of castor oil-based polyols with an average hydroxyl group functionali...
Embodiment 2
[0035] 1) Preparation of castor oil-based polyols with an average hydroxyl group functionality of 5.7
[0036] Add 9g of castor oil, 3.78g of 2-mercaptoethanol, 0.20g of photoinitiator 2,2-diethoxyacetophenone and 20ml of dichloromethane into the reactor, react under ultraviolet light for 8h at room temperature, and use deionized Excessive 2-mercaptoethanol was removed by water washing, the organic layer was dried with anhydrous magnesium sulfate, and methylene chloride was removed by rotary evaporation to obtain 9.77g of castor oil-based polyol with an average hydroxyl group functionality of 5.7, and the double bond reaction was determined to be complete by NMR technology. The yield was 87.1%.
[0037] 2) Preparation of castor oil-based branched polyolefins with an average carbon-carbon double bond functionality of 5.7
[0038] Add 4 g of castor oil-based polyols with an average hydroxyl group functionality of 5.7, 3.44 g of triethylamine and 30 ml of ethyl acetate into the ...
Embodiment 3
[0046]The preparation of the castor oil-based multi-branched cyclocarbonate whose cyclocarbonate average functionality is 6.8:
[0047] Add 7.48g of castor oil-based branched polyolefins with an average carbon-carbon double bond functionality of 8.7, 3.5g of mercapto cyclocarbonate, 0.15g of 2-hydroxy-2-methyl-1-phenylacetone and 10ml of chloroform Into the reactor, react under ultraviolet light at room temperature for 12 hours, and remove the solvent by rotary evaporation to obtain 10.98 g of castor oil-based polycyclic carbonate with an average functionality of cyclic carbonate of 6.8. Its NMR data are as follows:
[0048] 1 HNMR (400MHz, CDCl 3 , δppm) 5.82 (td, J = 14.3, 6.2Hz, 2.7H), 5.20 (s, 1H), 5.02 (dd, J = 18.4, 12.3Hz, 12H), 4.65 (dt, J = 15.4, 7.7Hz, 6H), 4.48-4.15(m, 25H), 4.03-3.85(m, 2H), 2.97(dd, J=20.1, 13.3Hz, 6H), 2.78(ddt, J=24.6, 12.6, 6.0Hz, 36H) ,2.54(t,J=7.3Hz,14H),2.42-2.23(m,17H),2.19-1.99(m,6H),1.87-1.73(m,5H),1.72-1.46(m,37H),1.27 (dd, J=14.4, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com