Method for synthesizing cefoxitin acid
A technology of cefoxitin acid and a synthesis method, which is applied in the field of synthesis of cefoxitin acid, can solve the problems of high cost of cefoxitin acid, reduced yield and the like, and achieves the advantages of reducing production steps, reducing production cost and low impurity content. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
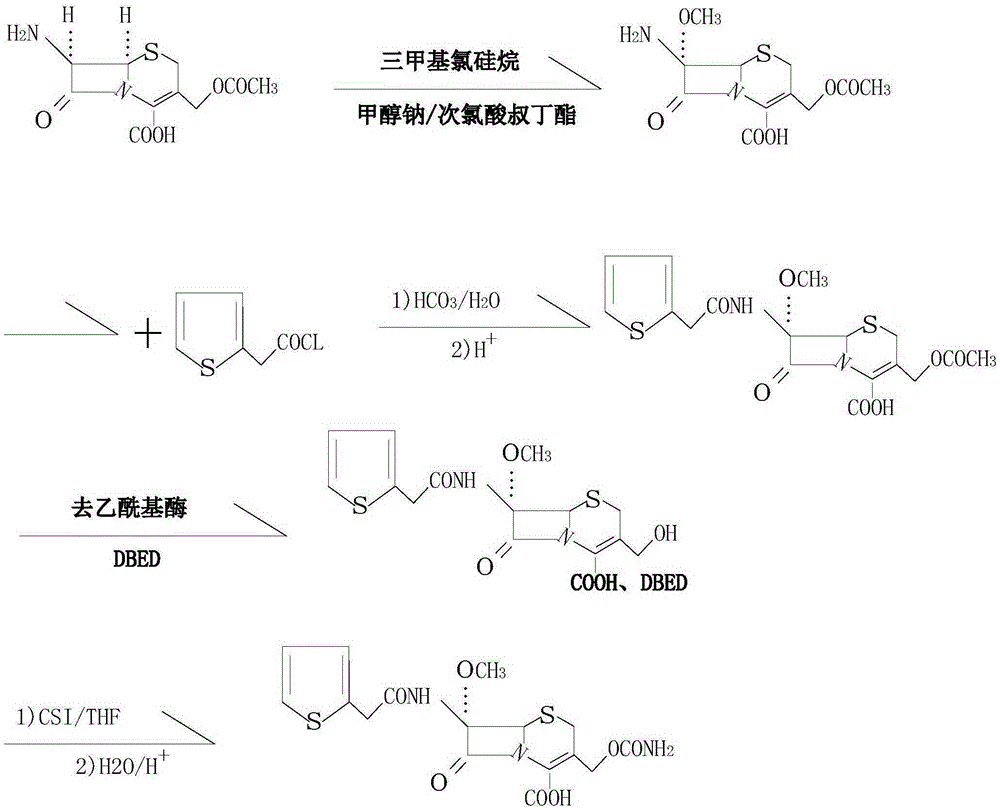
[0022] see figure 1 , in the embodiment of the present invention, a kind of synthetic method of cefoxitin acid, the steps are:
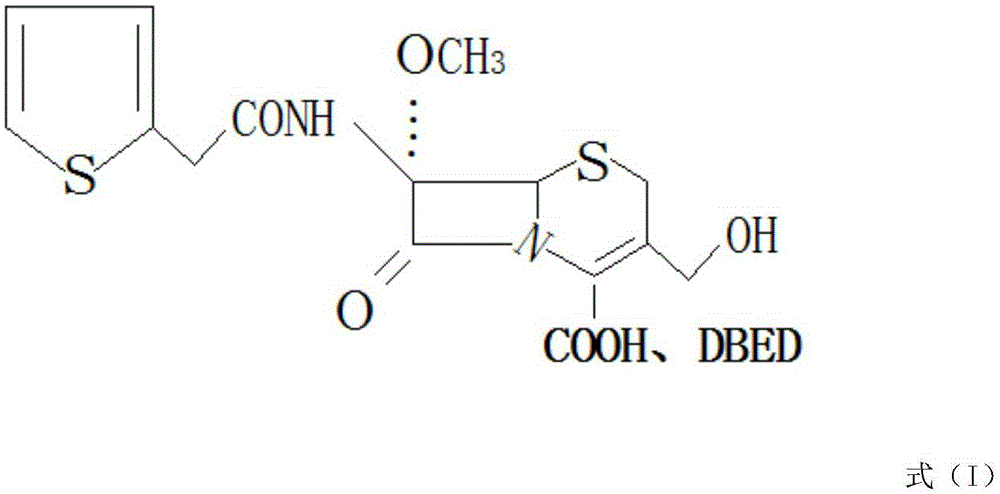
[0023] Use 7-aminocephalosporanic acid as raw material, add protective agents such as trimethylchlorosilane or BSA respectively, add sodium methoxide and tert-butyl hypochlorite after protection, and the molar ratio of 7-aminocephalosporanic acid to sodium methoxide 1:1. Under the catalytic condition of tert-butyl hypochlorite, react with sodium methoxide, the reaction temperature is -95°C, introduce a methoxyl group at the 7-position of 7-aminocephalosporanic acid to obtain a methoxyl compound, and the reaction ends after 50 minutes. Add 2-thiopheneacetyl chloride dropwise to react, then add deacylase to the obtained wet material in alkaline solution for hydrolysis, the hydrolysis temperature is 20°C, the hydrolysis time is 30min, after hydrolysis, filter out the acylase; after the reaction is completed, cool down to room temperature , drip benzat...
Embodiment 2
[0027] In the embodiment of the present invention, a kind of synthetic method of cefoxitin acid, the steps are:
[0028] Use 7-aminocephalosporanic acid as raw material, add protective agents such as trimethylchlorosilane or BSA respectively, add sodium methoxide and tert-butyl hypochlorite after protection, and the molar ratio of 7-aminocephalosporanic acid to sodium methoxide It is 1:2. Under the catalytic condition of tert-butyl hypochlorite, react with sodium methoxide, the reaction temperature is -60°C, introduce a methoxyl group at the 7-position of 7-aminocephalosporanic acid to obtain a methoxyl compound, and the reaction ends after 60 minutes. Add 2-thiophene acetyl chloride dropwise to react, then add deacylase to the obtained wet material in alkaline solution for hydrolysis, the hydrolysis temperature is 25°C, the hydrolysis time is 40min, after hydrolysis, filter out the acylase; after the reaction is completed, cool down to room temperature , drip benzathine diac...
Embodiment 3
[0032] In the embodiment of the present invention, a kind of synthetic method of cefoxitin acid, the steps are:
[0033] Use 7-aminocephalosporanic acid as raw material, add protective agents such as trimethylchlorosilane or BSA respectively, add sodium methoxide and tert-butyl hypochlorite after protection, and the molar ratio of 7-aminocephalosporanic acid to sodium methoxide It is 1:1.5. Under the catalytic condition of tert-butyl hypochlorite, react with sodium methoxide, the reaction temperature is -55°C, introduce a methoxyl group at the 7-position of 7-aminocephalosporanic acid to obtain a methoxyl compound, and the reaction ends after 55 minutes. Add 2-thiopheneacetyl chloride dropwise for reaction, then add deacylase to the obtained wet material in alkaline solution for hydrolysis, the hydrolysis temperature is 30°C, the hydrolysis time is 35min, after hydrolysis, filter out the acylase; after the reaction is completed, cool down to room temperature , drip benzathine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com