Culturing method of chick embryo forebrain neuron
A culture method, neuron technology, applied in the field of neuron culture, to achieve good responsiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
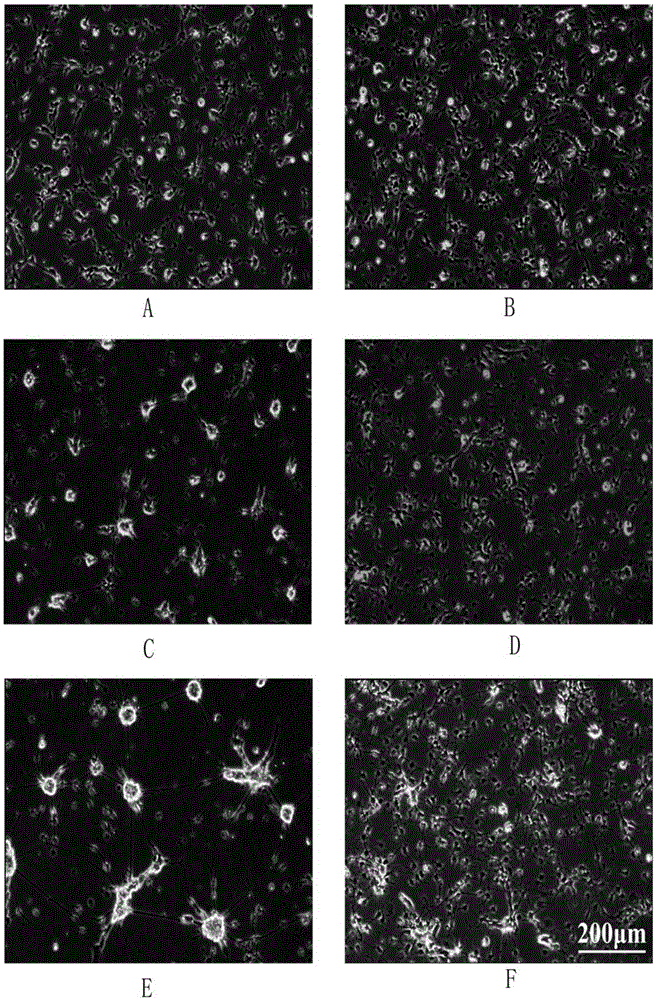
[0039] Nerve tissue dissection of chicken embryonic forebrain
[0040] (1) Take the fertilized eggs of white Laihang chickens from the 7th to the 9th day, spray them with 70% alcohol for disinfection, and put all the dissection instruments into the dissection ultra-clean bench;
[0041] (2) Crack the egg head (big head) with blunt medium-sized tweezers, and carefully remove the eggshell, peel off the shell membrane, take out the chicken embryo and put it in a 35mm Petri dish filled with ice-cold HBSS (Gibco);
[0042] (3) Cut off the head of the chicken embryo with No. 5 tweezers, and put it into another new 35mm Petri dish with HBSS;
[0043] (4) Put the back of the head of the chicken embryo upwards, press the midbrain with the No. 5 tweezers with the left hand, and uncover the meninges of the forebrain with the No. 5 tweezers with the right hand, and then clamp out the two groups of white tissues;
[0044] (5) Transfer the two groups of white tissues to a new 35mm culture ...
Embodiment 2
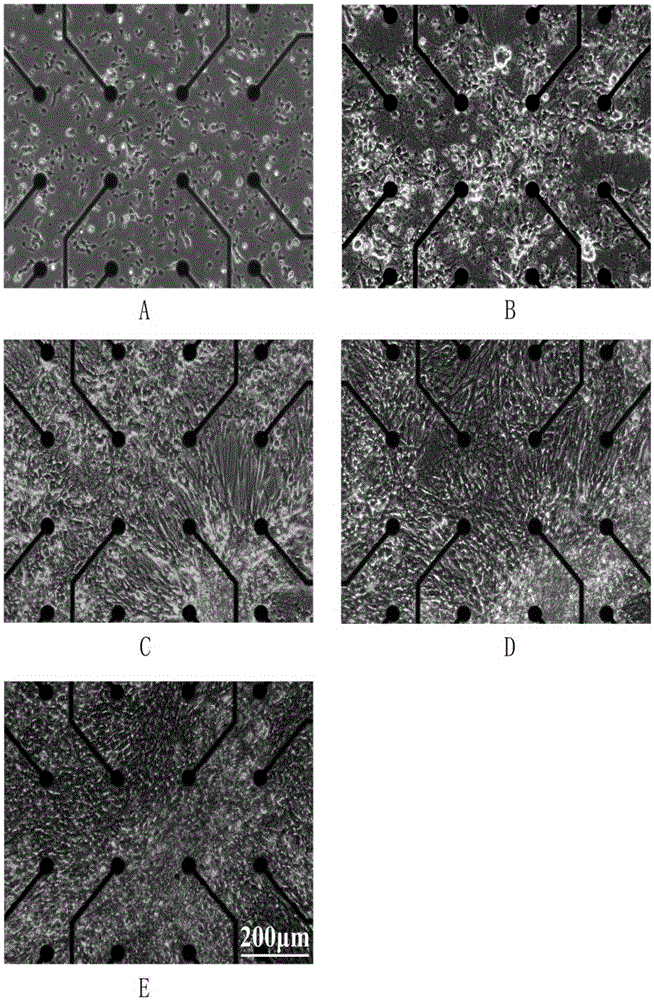
[0051] Microelectrode array culture of chick embryo forebrain neurons
[0052] (1) Surface treatment of microelectrode array (60MEA200 / 30iR-Ti, MultichannelSystem, Germany):
[0053] Washing: If the MEA that has been cultured is reused, first soak it in distilled water for 10 minutes to swell and rupture the cells, and then rinse off the surface cells with distilled water. Add 0.5 mL of trypsin solution and place at 37°C for 30 min to wash off excess cell debris. Then add neutral detergent water with enzymes, soak at room temperature overnight, then rinse with distilled water. If there are still very stubborn fragments, ultrasonicate for 3 minutes, and then rinse with distilled water. Finally rinse the surface twice with deionized water and let the surface dry.
[0054] Sterilization: UV sterilization for 30min.
[0055] Plasma treatment: put it into a plasma surface treatment instrument (PDC-32G, Harrick), and then evacuate it to below 200mTORR, inject a small amount of o...
Embodiment 3
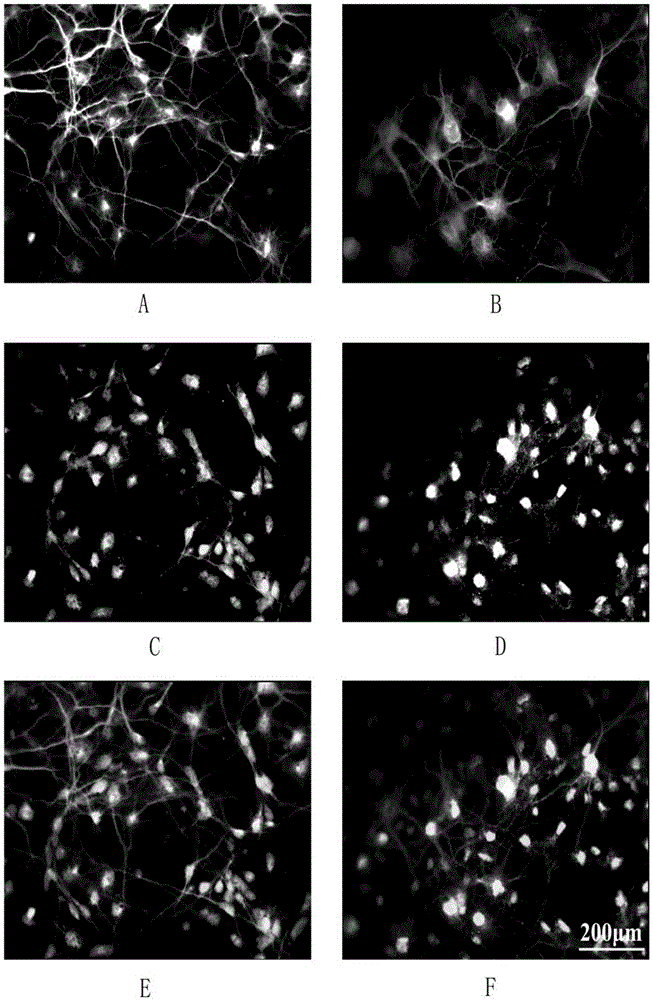
[0061] To confirm the cell type in the culture system, immunostaining was used to label neurons and glial cells for specific proteins. Among them, MAP2, microtubule-associated protein 2, is a neuron-specific skeletal protein, mainly distributed in the soma and dendrites. GFAP, glial fibrillary acidic protein, is a hallmark structural protein of glial cells. SynapsinI is a synapse-associated protein.
[0062] (1) Fixation: Wash the cell surface debris with PBS, and add 4% paraformaldehyde (containing 4% sucrose) for 15 minutes at room temperature.
[0063] (2) Membrane rupture: Aspirate the excess paraformaldehyde and wash it once with PBS. Add 1% Triton100 to break the membrane for 10min.
[0064] (3) Blocking: Aspirate the Triton100 permeabilization solution and wash it once with PBS. Add 1% albumin (BSA) to block non-specific groups.
[0065] (4) Primary antibody: Aspirate the blocking solution. Add the primary antibody combination for double staining according to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com