Preparation method of esomeprazole magnesium trihydrate for treating digestive system diseases
A technology for azole magnesium trihydrate and digestive system diseases, which is applied in the field of drug synthesis and achieves the effects of high reaction efficiency, simple and easy method, and good selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of esomeprazole magnesium trihydrate, comprising the following steps:
[0030]1) In the presence of sodium hydroxide, 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride 625g and 2-mercapto-5-methoxybenzimidazole 432g in 4LTHF Reflux reaction for 3 hours to obtain omeprazole sulfide, and then refine it. The refining process is: first dissolve omeprazole sulfide in 1.5L acetonitrile at 60°C, then drop into 3L petroleum ether, and dissolve it at 0.1°C Cool down to 10°C at a speed of 1 / s, let it stand for 1 hour, centrifuge and filter to obtain 650g of refined omeprazole sulfide, the yield is 85.9%, and the purity is 99.81%. 2-Chloromethyl-3,5-di The molar ratio of methyl-4-methoxypyridine hydrochloride to 2-mercapto-5-methoxybenzimidazole and sodium hydroxide is 1:0.8:0.1;
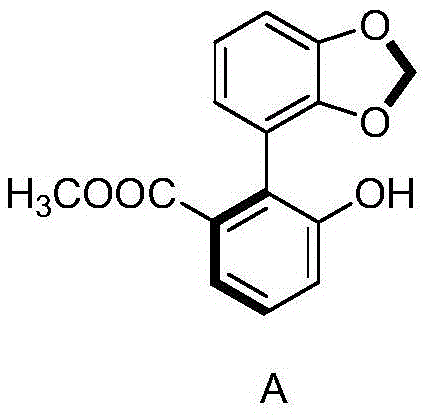
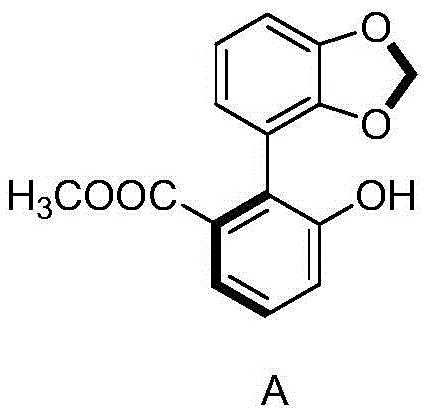
[0031] 2) Mix 630 g of omeprazole sulfide obtained in step 1) with the compound represented by formula A and the inorganic metal salt in 5 L of acetone. The mixing condit...
Embodiment 2
[0035] A preparation method of esomeprazole magnesium trihydrate, comprising the following steps:
[0036] 1) In the presence of potassium hydroxide, 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride 625g and 2-mercapto-5-methoxybenzimidazole 649g in 4LTHF reflux reaction for 4 hours to obtain omeprazole sulfide, and then refine it. The refining process is as follows: first dissolve omeprazole sulfide in 1.5L acetonitrile at 65°C, then drop 3.5L petroleum ether into 0.1 Cool down to 15°C at the speed of ℃ / s, let it stand for 1 hour, centrifuge and filter to obtain 845g of refined omeprazole sulfide, the yield is 89.1%, and the purity is 99.79%. Among them, 2-chloromethyl-3, The molar ratio of 5-dimethyl-4-methoxypyridine hydrochloride to 2-mercapto-5-methoxybenzimidazole and potassium hydroxide is 1:1.2:0.1;
[0037] 2) Mix 630 g of omeprazole sulfide obtained in step 1) with the compound represented by formula A and the inorganic metal salt in 5 L of acetone. The m...
Embodiment 3
[0041] A preparation method of esomeprazole magnesium trihydrate, comprising the following steps:
[0042] 1) In the presence of sodium carbonate, 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride 625g and 2-mercapto-5-methoxybenzimidazole 540g in 4LTHF Carry out reflux reaction for 4 hours to obtain omeprazole sulfide, and then refine it. The refining process is as follows: first dissolve omeprazole sulfide in 1.5L acetonitrile at 65°C, then drop 3.7L petroleum ether into the solution at 0.15°C Cool down to 12°C at a speed of 1 / s, let it stand for 1 hour, centrifuge and filter to obtain 790g of refined omeprazole sulfide, the yield is 83.5%, and the purity is 99.58%. 2-Chloromethyl-3,5-di The molar ratio of methyl-4-methoxypyridine hydrochloride to 2-mercapto-5-methoxybenzimidazole and sodium carbonate is 1:1:0.15;
[0043] 2) Mix 630 g of omeprazole sulfide obtained in step 1) with the compound represented by formula A and the inorganic metal salt in 5 L of aceton...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com