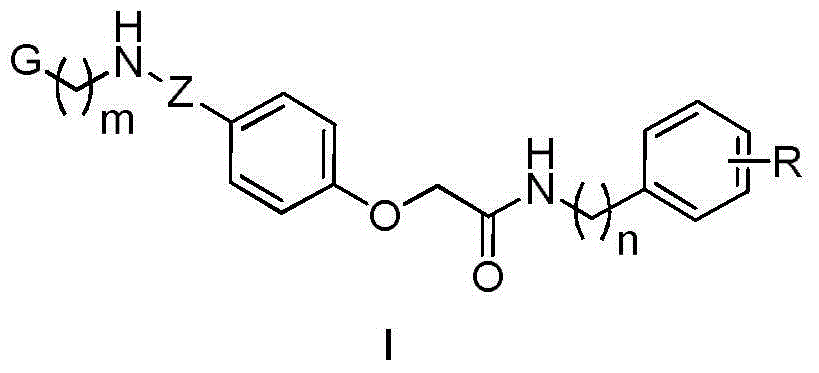
4-aminoacylphenoxyacetamide compound and medicine uses thereof
A technology of aminoacylphenoxyacetamide and compound, which is applied in the field of medicinal chemistry, can solve the problems of short half-life, poor water solubility and stability and other physical and chemical properties, weak inhibitory effect, etc., and achieve small toxic and side effects, good potential and application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
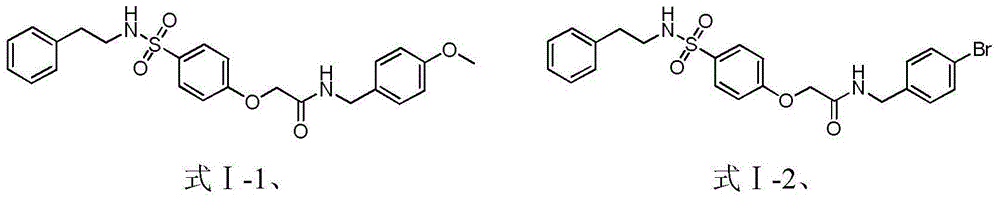
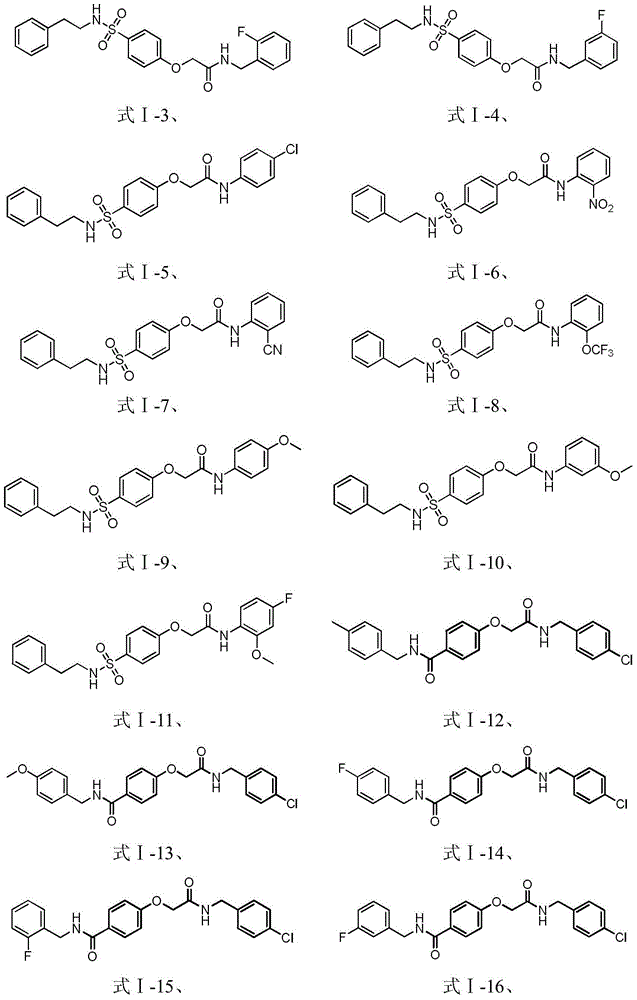
[0045] Example 1: Preparation of N-(4-chlorophenyl)-2-(4-(N-phenylethylsulfamoyl) phenoxy)acetamide (formula Ⅰ-5)
[0046] One, the synthesis of 4-hydroxyphenyl-1-sulfonyl chloride (compound 4)
[0047]
[0048] Dissolve 1.96g (10mmol, 1.0eq) of sodium p-hydroxybenzenesulfonate in 3.7ml of thionyl chloride, add 0.06ml of DMF, and react at 60°C for 3.5h, then pour the reaction solution into ice water and stir 5min, extracted with dichloromethane, washed the organic phase with saturated brine, anhydrous Na 2 SO 4 After drying and concentrating, 1.93 g of a crude product of colorless transparent oil was obtained, which could be directly used in the next reaction without purification. MS(ESI)(m / z):193.0(M+H) + .
[0049] Two, the synthesis of 4-hydroxyl-N-phenethylbenzenesulfonamide (compound 6)
[0050]
[0051] 1.21g (10mmol, 1.0eq) of phenethylamine was dissolved in 5ml of dichloromethane, and 1.6ml of pyridine was added dropwise thereto. Under ice bath, 1.93g (10mm...
Embodiment 2
[0060] Embodiment 2: compound formula I-1, I-2, I-3, I-4, I-6, I-7, I-8, I-9, I-10, I-11 synthesis
[0061] 1. Synthesis of intermediate compounds 2-a~2-d and 2-f~2-k
[0062]
[0063]
[0064] Referring to the conditions for the synthesis of compound 2-e in the third step in Example 1, intermediate compounds 2-a~2-d and 2-f~2-k were prepared from substituted benzylamine or aniline and chloroacetyl chloride.
[0065] Two, compound formula I-1, I-2, I-3, I-4, I-6, I-7, I-8, I-9, I-10, I-11 synthesis
[0066]
[0067]
[0068] With reference to the conditions of the fourth step synthesis compound formula I-5 in the embodiment example 1, from 4-hydroxyl-N-phenylethylbenzenesulfonamide (compound 6) and intermediate compounds 2-a~2-d and 2-f~ 2-k obtains compound formula I-1 to I-4 and formula I-6 to I-11, specifically: N-(4-methoxybenzyl)-2-(4-(N-phenethylamine Sulfonyl)phenoxy)acetamide (formula Ⅰ-1); N-(4-bromobenzyl)-2-(4-(N-phenethylsulfamoyl)phenoxy)acetamide (f...
Embodiment 3
[0080] Example 3: Preparation of 4-(2-((4-chlorobenzyl)amino)-2-oxoethoxy)-N-(4-methylbenzyl)benzamide (formula Ⅰ-12)
[0081] 1. Synthetic intermediate 2-chloro-N-(4-chlorobenzyl)acetamide (compound 2-1)
[0082]
[0083] 5.00g (35mmol, 1.0eq) of 4-chlorobenzylamine was added to a mixed solution of 5.6ml of pyridine and 70ml of water, and 5.90g (52.5mmol, 1.5eq) of chloroacetyl chloride was added dropwise under ice-bath conditions. React at 0°C for 1.0 h, add 175 ml of water to the system, filter, filter out the solid and dry to obtain 6.50 g of a light yellow solid with a yield of 84.4%. The crude product can be carried out in the next step without purification.
[0084] Two, synthetic 4-hydroxybenzoic acid methyl ester (compound 8)
[0085]
[0086] Add 10.00 g (72 mmol) of 4-hydroxybenzoic acid (compound 7) and 20 mL of methanol to the reaction flask, and add 6.8 mL (94 mmol) of thionyl chloride dropwise to the above system under ice cooling. Afterwards, the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com