A thermophilic alkaline pectin lyase gene, engineering bacteria, thermophilic alkaline pectin lyase and application thereof
A technology of pectin lyase and genetically engineered bacteria, which is applied in the field of bioengineering, can solve the problems of few reports on thermophilic pectinase, and achieve the effect of increasing fermentation capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Construction of thermophilic engineering bacteria and expression of enzymes
[0036] (1) Primer design and acquisition of thermophilic alkaline pectin lyase gene PelC by PCR method
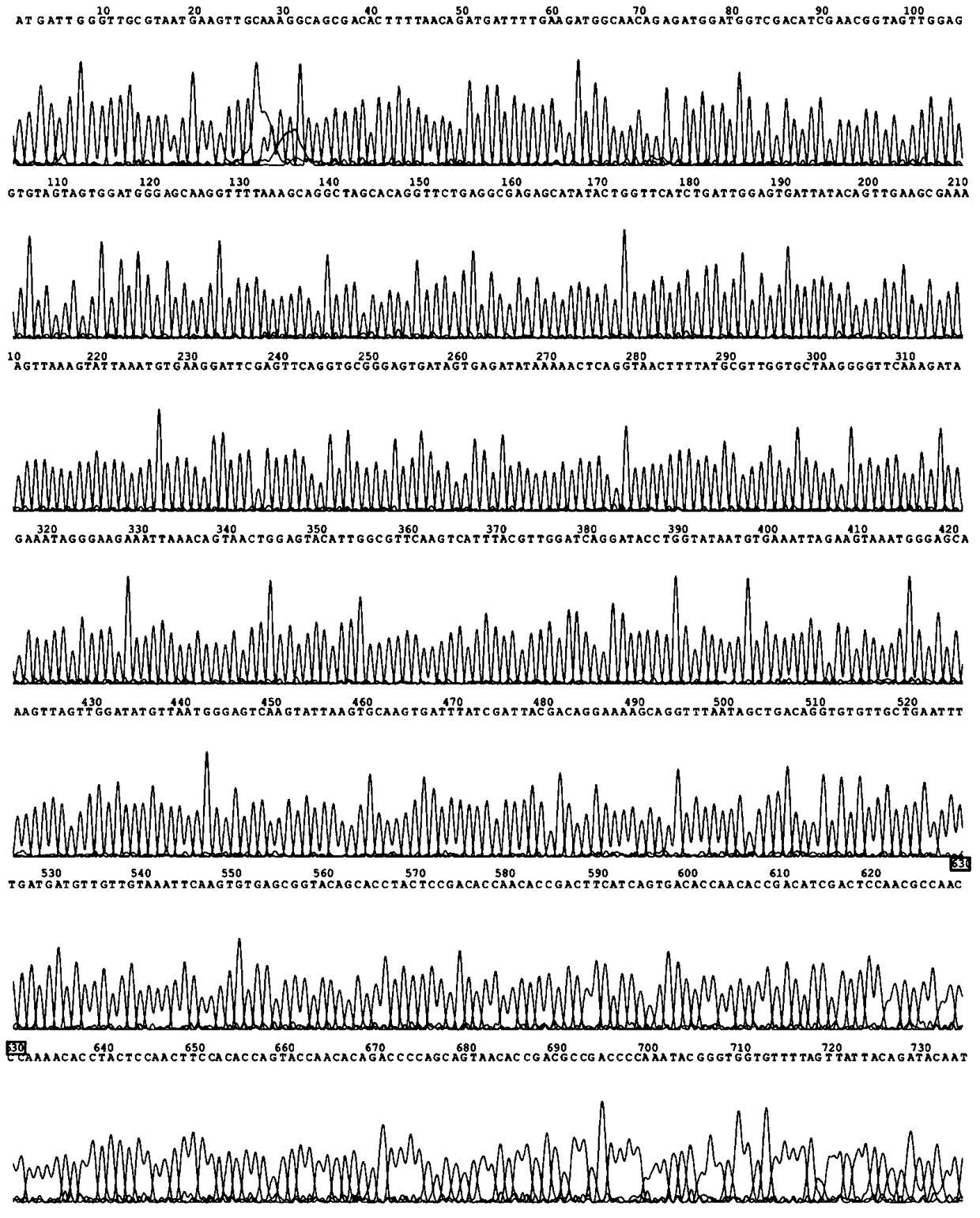
[0037]Using the genome of Fusobacterium caldicellulosiruptor bescii DSM6725 as a template, primers were designed to amplify the PelC gene by PCR, and the amplified product was subjected to agarose gel electrophoresis, and 1300 bp was recovered and purified. Sequencing showed that the size of the fragment was 1305 bp, and its sequence As shown in SEQ ID NO.1.
[0038] The sequences of the upstream primers are as follows:
[0039] Upstream Primer 1:
[0040] 5'GGAATTC CATATG ATTGGGTTGCGTAATGAAGTTGCAAAGGCAGC 3'
[0041] (The underlined base is the recognition sequence of NdeI digestion);
[0042] The sequences of the downstream primers are as follows:
[0043] Downstream primer 2:
[0044] 5'CCG CTCGAG TCAGTGGTGGTGGTGGTGGTGCTCGAGGTATTGATG 3'
[0045] (The bases underlined ...
Embodiment 2
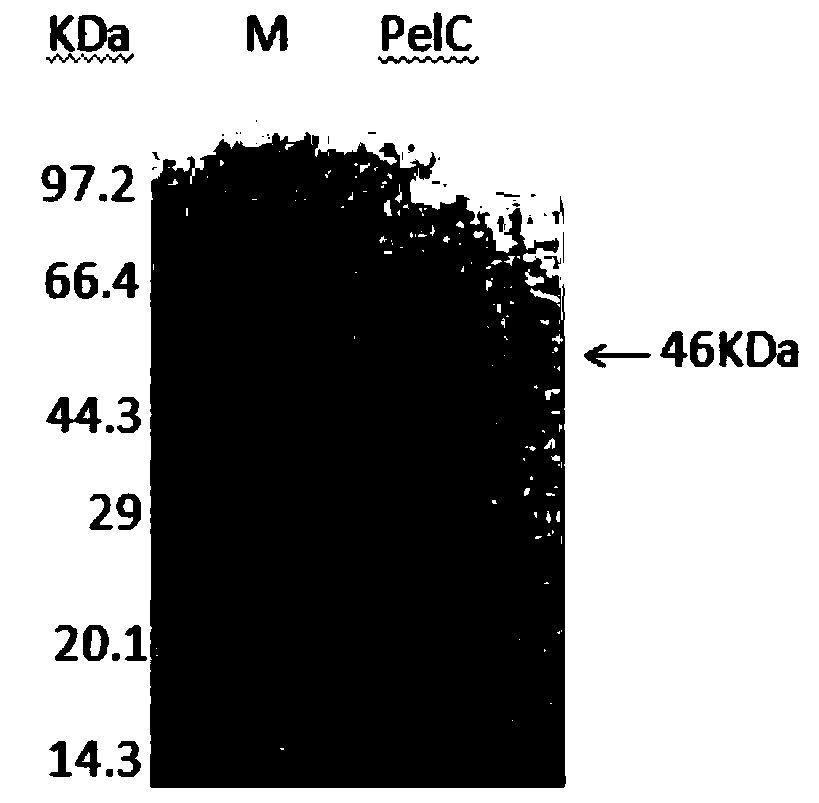
[0062] Embodiment 2: Characteristic of recombinant thermophilic alkaline pectin lyase
[0063] The enzyme activity assay method of thermophilic alkaline pectin lyase described in embodiment 1 is specifically as follows:
[0064] Substrate preparation: Weigh 0.2g of polygalacturonic acid and place it in a 100mL beaker, add about 80mL of 50mM Gly and stir to dissolve, adjust the pH to 9.5 with 1mol / L NaOH, and dilute the solution with a volumetric flask to 100mL, dubbed as 0.2% (w / v) substrate.
[0065] Control group: add 100μL, 0.8mM, CaCl to 800μL substrate 2 , preheat at 70°C for 1 min, add 100 μL, 20 mM, pH 8.0 Tris-HCl, and measure the absorbance at 235 nm within 2 min;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com