Electric conduction nanometer copper ink preparation method
A technology of conducting nanometer and nanometer copper, applied in the field of nanomaterials, can solve the problems of narrow temperature range, high cost and high cost of reducing agent L-ascorbic acid, and achieve the effects of high reactivity, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
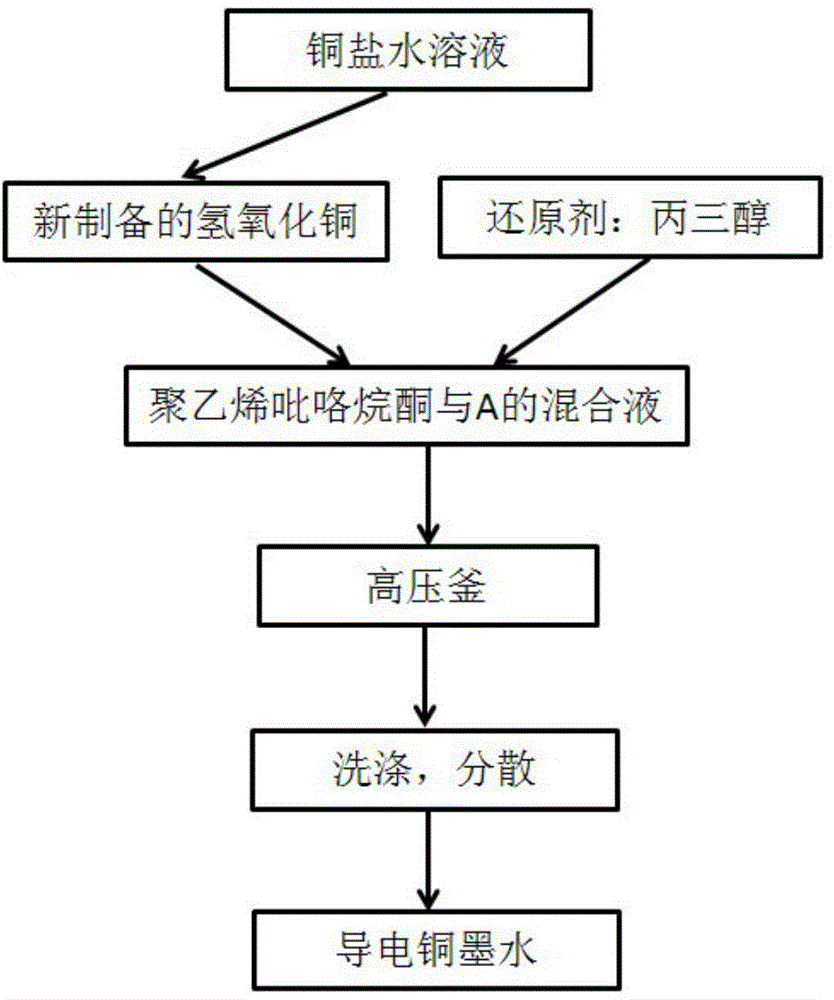
[0044] (1) Dissolve 0.05 mol of copper sulfate in 100 ml of distilled water, add dropwise 30 ml of ammonia water with a volume ratio of 1:1 under stirring at room temperature, until the blue precipitate is just completely dissolved, and the solution turns dark blue;
[0045] (2) Slowly add 50ml of 0.1mol sodium hydroxide solution to the above solution to form a blue copper hydroxide precipitate, wash with deionized water and absolute ethanol three times respectively, and then freshly prepared copper hydroxide can be obtained ;
[0046] (3) Add 1.9g of polyvinylpyrrolidone K-30, 0.3g of newly prepared copper hydroxide and 5M~6.6M reducing agent glycerol to 30ml of absolute ethanol in sequence;
[0047] (4) Add the mixed solution into a polytetrafluoroethylene autoclave tank, and react for 20-24 hours at 80-100°C to obtain a nano-copper dispersion;
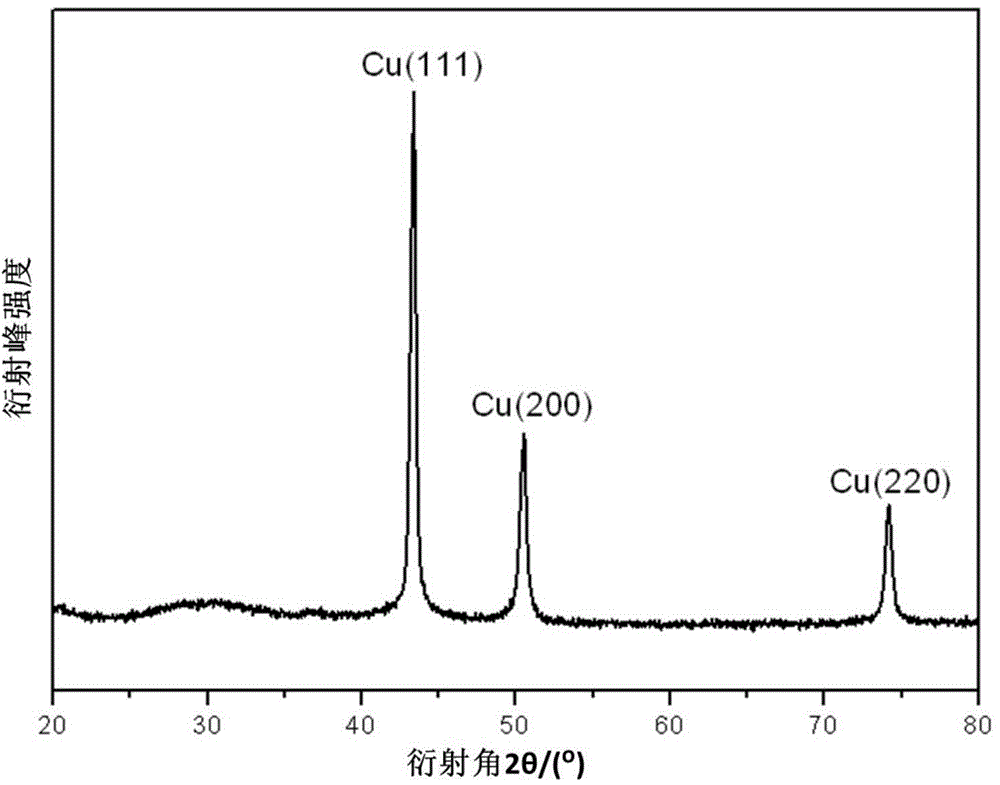
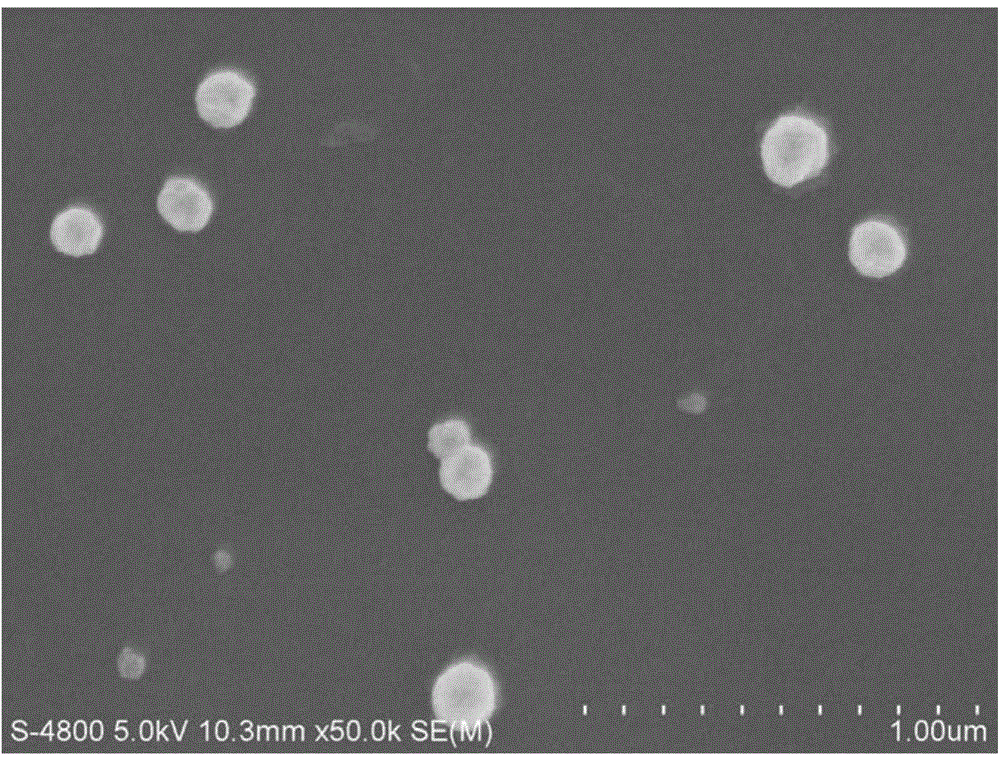
[0048] (5) Centrifuge the nano-copper dispersion at 8000~10000 rpm for 20~30 minutes, wash it with absolute ethanol for 3 times, ...
Embodiment 2
[0051](1) Dissolve 0.05mol of copper chloride in 100ml of distilled water, and add dropwise 30ml of ammonia water with a volume ratio of 1:1 under normal temperature stirring conditions until the blue precipitate is completely dissolved and the solution turns dark blue.
[0052] (2) Slowly add 50ml of 0.1mol sodium hydroxide solution to the above solution to form a blue copper hydroxide precipitate, wash with deionized water and absolute ethanol three times respectively, and then freshly prepared copper hydroxide can be obtained ;
[0053] (3) Add 12g of polyvinylpyrrolidone K-15, 0.6g of newly prepared copper hydroxide and 3.3M~5M reducing agent glycerol to 30ml of deionized water in sequence;
[0054] (4) Add the mixed solution into a polytetrafluoroethylene autoclave tank, and react for 12-20 hours at 100-150°C to obtain a nano-copper dispersion;
[0055] (5) Centrifuge the nano-copper dispersion at a speed of 10000~12000 rpm for 10~20 minutes, wash it with deionized water...
Embodiment 3
[0058] (1) Dissolve 0.05mol of copper nitrate in 100ml of distilled water, add dropwise 30ml of ammonia water with a volume ratio of 1:1 under stirring at room temperature, until the blue precipitate is completely dissolved and the solution turns dark blue.
[0059] (2) Slowly add 50ml of 0.1mol sodium hydroxide solution to the above solution to form a blue copper hydroxide precipitate, wash with deionized water and absolute ethanol three times respectively, and then freshly prepared copper hydroxide can be obtained ;
[0060] (3) Add 2.2g of polyvinylpyrrolidone K-60, 0.44g of freshly prepared copper hydroxide and 1.7M~3.3M reducing agent glycerol to 30ml of ethylene glycol in sequence;
[0061] (4) Add the mixed solution into a polytetrafluoroethylene autoclave tank, and react for 6-12 hours at 150-200°C to obtain a nano-copper dispersion;
[0062] (5) Centrifuge the nano-copper dispersion at a speed of 12000-15000 rpm for 5-10 minutes, wash it with acetone for 3 times, pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com