Method for regenerating ATP(adenosine triphosphate) through enzyme method
A polyphosphate kinase and bacillus technology, which is applied in biochemical equipment and methods, enzymes, transferases, etc., can solve the problems that the enzyme coupling reaction cannot be carried out at room temperature, cannot widely meet the needs of industrial production, and the degree of polyphosphate polymerization is high. , to reduce costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
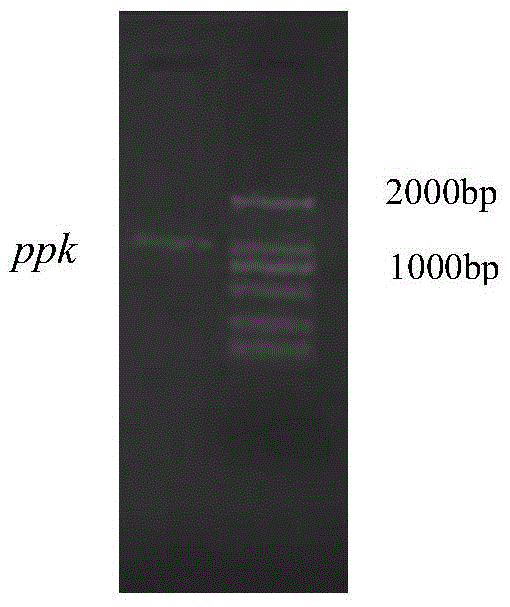
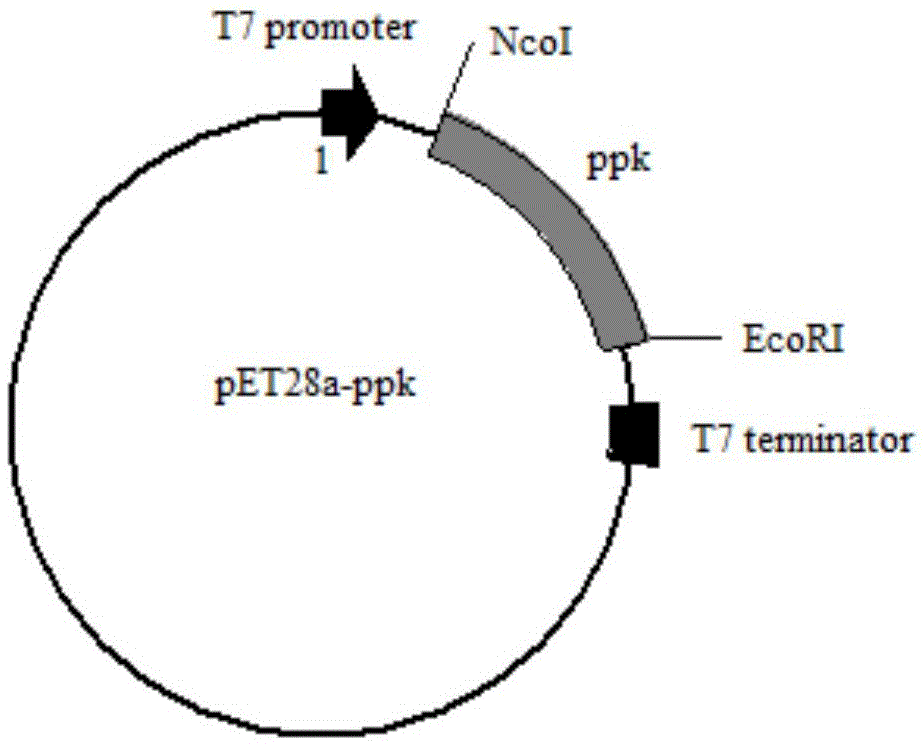
[0031] 1.1 Cloning of ppk polyphosphate kinase gene in Corynebacterium glutamicum strain and construction of recombinant vector
[0032] The ppk polyphosphokinase gene in the Corynebacterium glutamicum strain was synthesized by genome amplification method. Using the whole genome of the strain as a template, primers were designed according to the GenBank sequence, and corresponding restriction sites (NcoI and EcoRI) and protective bases were added.
[0033] The primers used for PCR amplification of polyphosphate kinase gene ppk are as follows:
[0034] Upstream primer: 5'-GCATATGATGACCGCCACCGATTC-3'
[0035] Downstream primer: 5'-GAAGCTTCCGGTTGGTAGCTGTGAC-3'
[0036] The PCR system reaction system contains 0.2 μL of genomic DNA, 0.5 μL of upstream and downstream primers, 10 μL of Extaq mixture, and added double distilled water to make up to 20 μL. The reaction conditions were pre-denaturation at 94°C for 5 min, denaturation at 94°C for 30 s, annealing at 55°C for 30 s, exten...
Embodiment 2
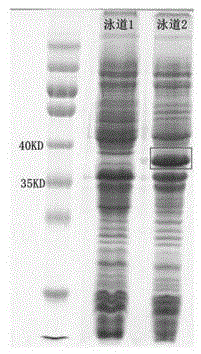
[0040] Transfer the constructed expression vector pET28a-ppk plasmid into BL21(DE3) Escherichia coli, use LB medium after picking the bacteria, grow at 37°C until the OD600 is about 0.4-1.0, and induce with 0.1mM-1mM IPTG , for intracellular expression at 30°C. The vector pET28a was transformed into BL21(DE3) Escherichia coli, and the expression was induced and expressed under the same conditions as above, and the expressed crude enzyme solution was subjected to protein electrophoresis to verify the expression result. The protein electrophoresis was as follows: image 3 As shown, the protein content measured by the Coomassie brilliant blue method was 2.3 g / L.
Embodiment 3
[0042] Three experimental groups were set up in reaction system 1. The phosphoric acid donors in the experimental groups were tripolyphosphate, tetrapolyphosphate and hexametaphosphate respectively. 1mMADP, 30mMMgCl 2 , 2mM polyphosphate, 250ul PPK crude enzyme solution (containing 0.547mg of protein), add 3M pH8.0 Tris-HCl10ul to adjust the pH to 8.0, system 1 reacted at 37 degrees for 5 minutes, placed in a 60 degrees water bath and heated for 20 minutes to inactivate the PPK enzyme . Take the supernatant and add it to NADPH reaction system 2. System 2 includes 1mM glucose, 1mM NADP, 100ul hexokinase (1U), 100ul 6-phosphate glucose dehydrogenase (1U). System 2 measures NADPH at 340nm after reacting at 30°C for 30min According to the absorbance of the molar absorptivity, the amount of NADPH generated was calculated. The PPK enzyme activities measured with the three phosphate substrates were 0.162umol / min / ml, 0.176umol / min / ml, and 0.193umol / min / ml, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com