Method for preparing aromatic aldehyde/ketone compound based on photoelectrocatalysis
A technology of photoelectric catalysis and ketone compounds, which is applied in the field of synthesis of aromatic aldehyde/ketone compounds to achieve the effects of reducing pollution, mild reaction conditions, and low requirements for reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] reaction system:
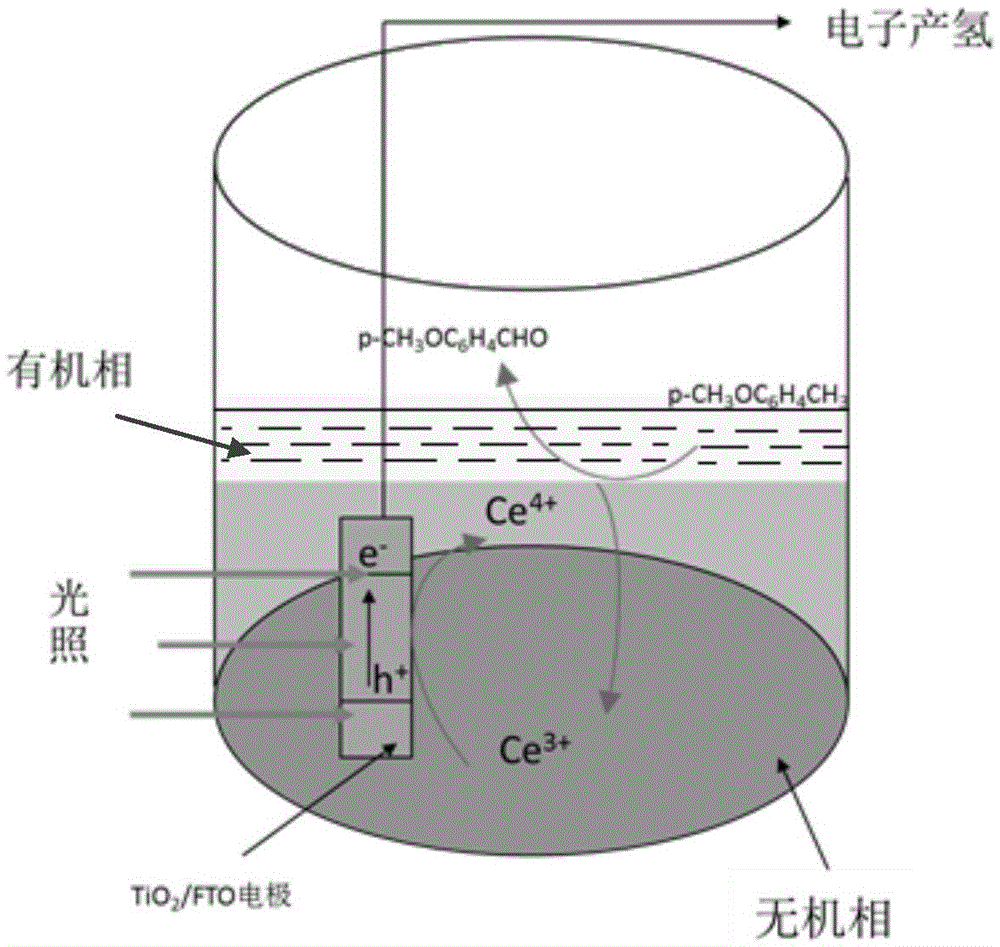
[0038] Anolyte: mix 5uL p-methylanisole with 65mL of 0.12mol / L Ce 2 (SO 4 ) 3 Nitric acid solution (nitric acid concentration 1.0mol / L) mixed; wherein, 0.12mol / L of Ce 2 (SO 4 ) 3 The preparation method of the nitric acid solution is as follows: at 25° C., weigh 68.2 g of cerous sulfate octahydrate, and measure 69 ml of concentrated nitric acid (65 wt %) to prepare a 1.0 L aqueous solution.
[0039] Catholyte: 1.0mol / L nitric acid solution;
[0040] Anode: TiO 2 / FTO thin film electrode;
[0041] TiO 2 / Preparation of FTO film electrode: Mix 30mL ultrapure water and 30mL concentrated hydrochloric acid, then add 2.0mL isopropyl titanate dropwise and transfer them to a hydrothermal kettle together, then put the washed FTO glass slide. The hydrothermal kettle was hydrothermally heated at 155°C for 4 hours. After cooling, take out the FTO and wash it, and heat it in a muffle furnace at 200°C for 2 hours to obtain TiO 2 / FTO.
[0042] Cathode: ...
Embodiment 2
[0048] reaction system:
[0049] Anolyte: mix 5uL p-chlorotoluene with 65mL of 0.12mol / L Ce 2 (SO 4 ) 3 Nitric acid solution mixed (nitric acid concentration 1.0mol / L);
[0050] Catholyte: 1.0mol / L nitric acid solution;
[0051] Anode: TiO 2 / FTO thin film electrode;
[0052] Cathode: Pt;
[0053] Reference electrode: mercury-mercurous sulfate;
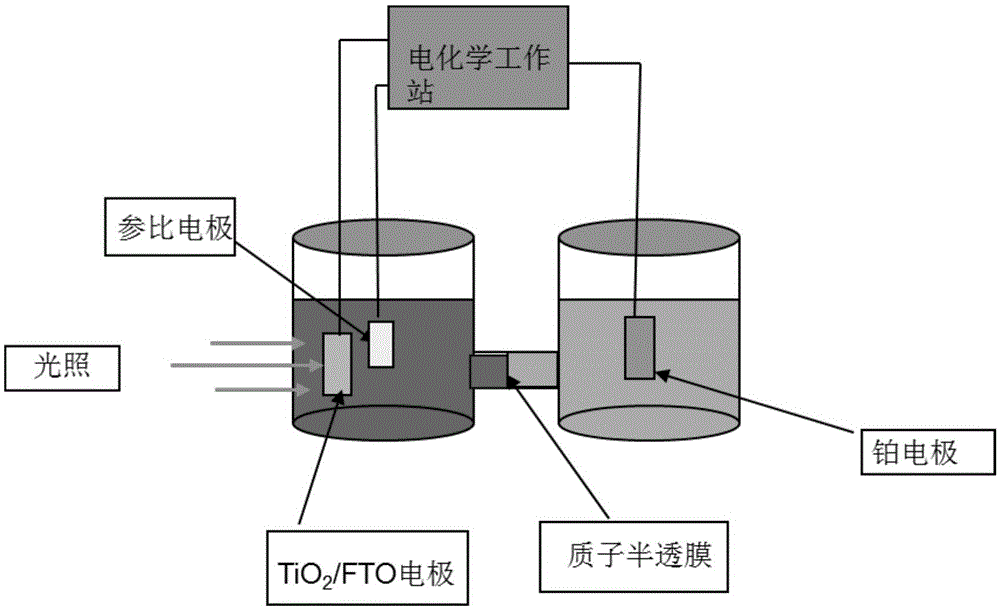
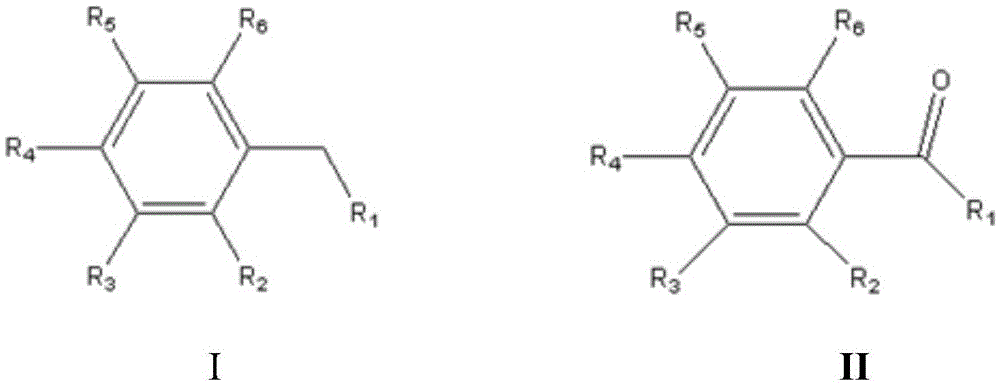
[0054] Reactor such as figure 2 As shown, the cathode was exhausted with nitrogen gas for 10 minutes, and the above reaction system was subjected to a bias voltage of 0.7V, a reaction temperature of 25°C, a light source of 300W xenon lamp to simulate sunlight, and a reaction time of 2 hours. After the reaction, the anolyte was extracted, and the organic phase was purified to obtain p-chlorobenzaldehyde (structural formula is shown below), the inorganic phase was recycled, and the cathode product was hydrogen.
[0055] Structural formula:
[0056]
Embodiment 3
[0058] reaction system:
[0059] Anolyte: mix 5uL ethylbenzene with 65mL of 0.12mol / L Ce 2 (SO 4 ) 3 Nitric acid solution mixed (nitric acid concentration 1.0mol / L);
[0060] Catholyte: 1.0mol / L nitric acid solution;
[0061] Anode: TiO 2 / FTO thin film electrode;
[0062] Cathode: Pt;
[0063] Reference electrode: mercury-mercurous sulfate;
[0064] Reactor such as figure 2 As shown, the cathode was exhausted with nitrogen gas for 10 minutes, and the above reaction system was subjected to a bias voltage of 0.7V, a reaction temperature of 25°C, a light source of 300W xenon lamp to simulate sunlight, and a reaction time of 2 hours. After the reaction, the anolyte is extracted, the organic phase is purified to obtain acetophenone (the structural formula is shown below), the inorganic phase is recycled, and the cathode product is hydrogen.
[0065] Structural formula:
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com