Application of platinum catalyst in preparation of propylene by dimethylmethane
A platinum catalyst and a catalyst technology, applied in the field of Pt catalyst and its preparation, can solve the problems of easy deactivation selectivity, fast deactivation rate, carbon deposition of platinum-based catalysts, etc. The effect of propylene selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Dissolve 8.7g of aluminum sec-butoxide (ATSB), 0.86g of butyl titanate (TTB) and 0.75g of CTAB in 12g of isopropanol, and stir with the rotor for 2 hours;
[0030] (2) Mix 0.705g of 65wt% concentrated nitric acid and 12.5g of deionized water, add the mixed solution dropwise to the precursor solution for hydrolysis, and the hydrolysis time is 0.5h;
[0031] (3) Leave the resulting gel to age for 24 hours, then remove the solvent, dry at 70°C for 20 hours, and bake at 600°C for 3 hours to obtain TiO 2 -Al 2 o 3 Composite carrier;
[0032] (4) TiO 2 -Al 2 o 3 The composite carrier is immersed in a chloroplatinic acid solution with a concentration of 0.01g / ml, ultrasonicated for 0.5h, and dried at room temperature for 12h;
[0033] (5) Drying at 90°C for 12h, and calcination at 600°C for 3h, the obtained PtO 2 / TiO 2 -Al 2 o 3 Catalyst; finally in H 2 Reduction under atmosphere for 1h to obtain Pt / TiO 2 -Al 2 o 3 catalyst.
[0034] Obtained TiO 2 -Al 2 ...
Embodiment 2
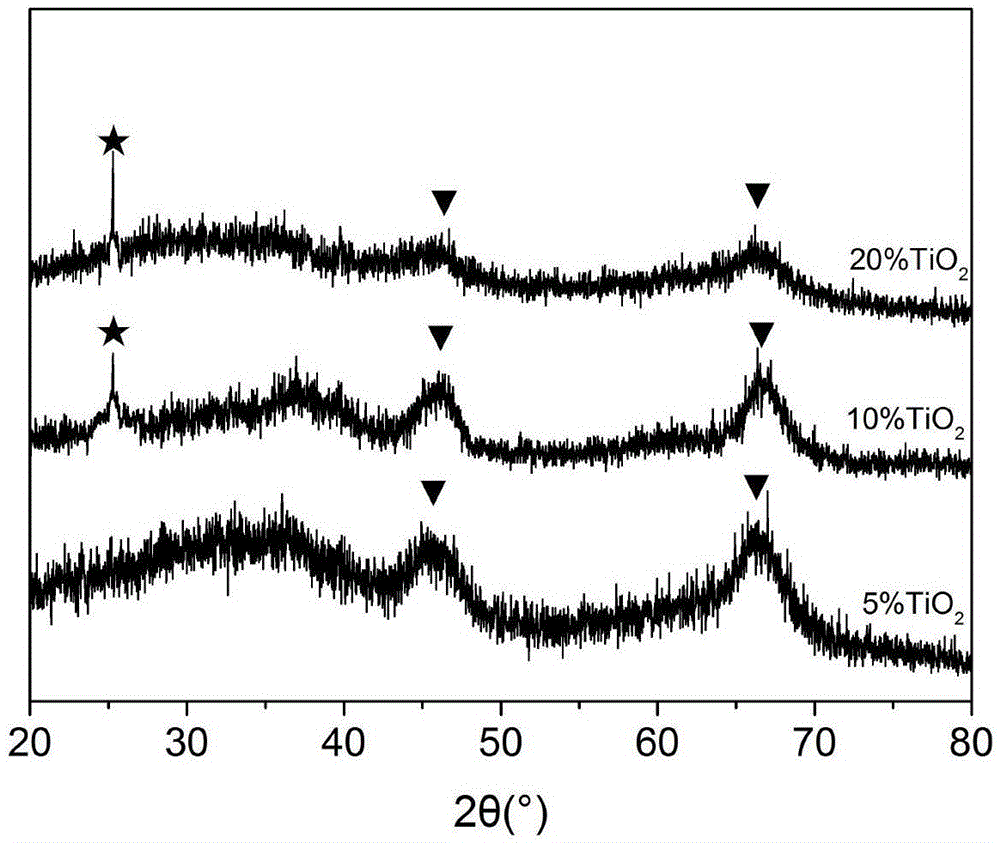
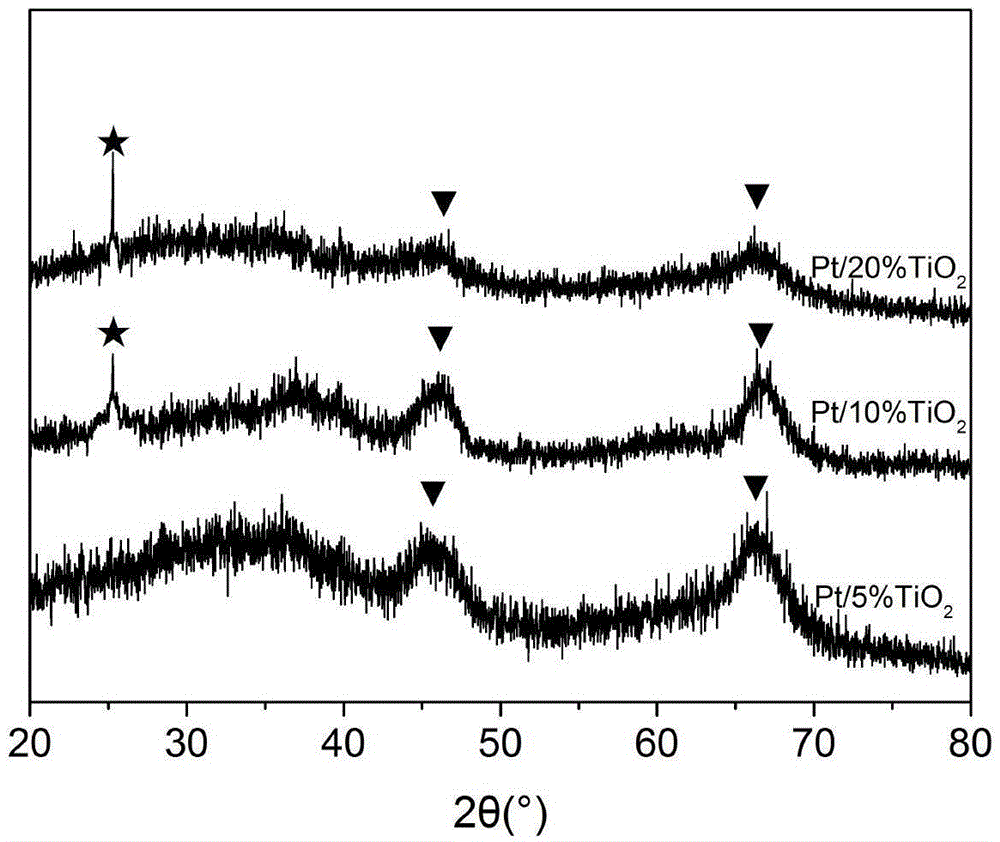
[0048] Adopt embodiment 1 method to react, its difference is only that in the step (1) aluminum sec-butoxide (ATSB) consumption is 9.2g, and butyl titanate (TTB) consumption is 0.43g, in the gained catalyst TiO 2 The mass percentage content is 5%. The resulting TiO 2 -Al 2 o 3 The XRD spectrum of the carrier is as figure 1 As shown, the obtained Pt / TiO after reduction 2 -Al 2 o 3 The XRD spectrum of the catalyst is as figure 2 shown.
Embodiment 3
[0050] Adopt embodiment 1 method to react, and its difference is only that in the step (1) aluminum sec-butoxide (ATSB) consumption is 7.7g, and butyl titanate (TTB) consumption is 1.72g, and in the gained catalyst TiO 2 The mass percentage is 20%. Obtained TiO 2 -Al 2 o 3 The XRD spectrum of the carrier is as figure 1 As shown, the obtained Pt / TiO after reduction 2 -Al 2 o 3 The XRD spectrum of the catalyst is as figure 2 shown.
[0051] from figure 1 It can be seen that the composition of the obtained support is TiO 2 and Al 2 o 3 , verified as a double oxide composite support; when TiO 2 At 5% content, no TiO 2 peak appears when TiO 2 When the mass percentage reaches 10%, TiO appears 2 Diffraction peaks and their peak intensity varies with TiO 2 The mass percentage of Al increases and continuously strengthens, while Al 2 o 3 The intensity of the peak decreases continuously, thus proving that the TiO 2 Distributed in Al 2 o 3 surface and are highly disp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com