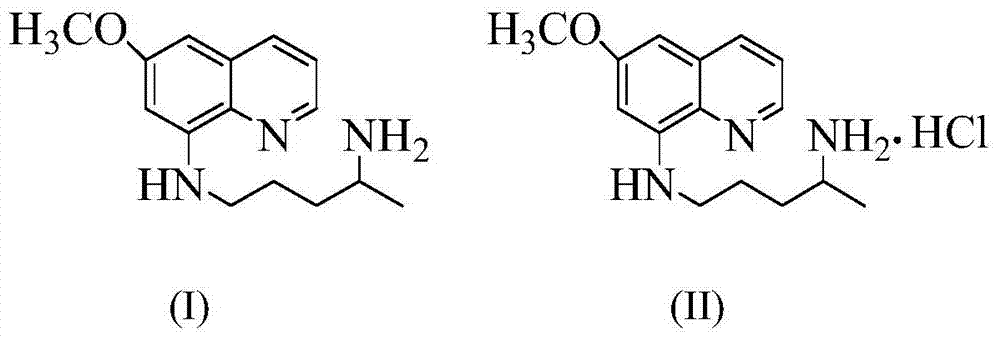
A kind of preparation method of quinoxide and its hydrochloride
A technology of hydrochloride and methyl acrylate, applied in the field of preparation of quinacetate and its hydrochloride, can solve problems such as curative effect discount, no domestic and foreign literature reports on the synthesis route, etc., and achieves stable quality, simple post-processing, Less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
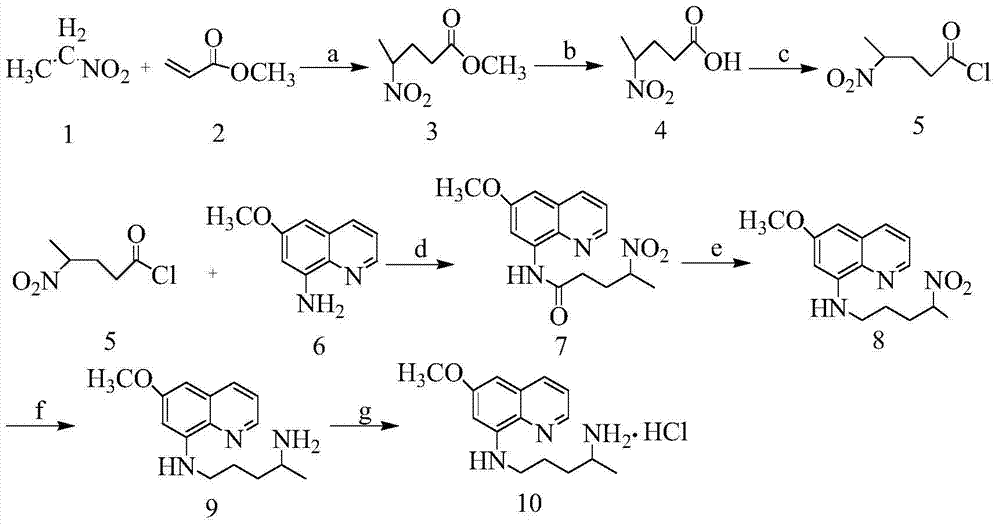
Embodiment 1
[0023] (1) Synthesis of 4-nitro-methyl pentanoate (compound 3):
[0024] Take nitroethane (compound 1) (225.2g, 3mol), methyl acrylate (compound 2) (86.1g, 1mol) and triethylamine (50.5g, 0.5mol), heat up to 30°C, and continue to stir for reaction 3 day, and then the solvent was distilled off under reduced pressure to obtain viscous compound 3 (104.6 g, 65%). 1 HNMR (CDCl 3 )δ (ppm): 4.631-4.669 (m, 1H, CH), 3.696 (s, 3H, CH 3 O),2.300-2.416(m,2H,CH 2 ),2.058-2.285(m,2H,CH 2 ),1.562-1.579(m,3H,CH 3 ).
[0025] (2) Synthesis of 4-nitro-pentanoic acid (compound 4):
[0026] Add 6mol / L hydrochloric acid (300mL) to compound 3, the product of the previous step, heat to reflux for 4 hours, cool to room temperature, extract with ethyl acetate (3x500mL), combine the organic phases and wash with saturated sodium bicarbonate, and add hydrochloric acid dropwise to the combined aqueous phases to neutralize PH=2, extracted with ethyl acetate (3x500mL), dried over anhydrous sodium su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com