Method for catalyzed synthesis of cyclic carbonate through functional metal organic frame material
A technology of metal-organic frameworks and cyclic carbonates, applied in organic chemistry, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc. Air and water sensitive issues, to achieve excellent catalytic activity, good reusability, high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
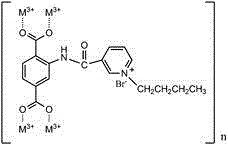
[0027] Catalyst preparation: NH 2 -MIL-101(Cr), nicotinoyl chloride and pyridine are added to N,N-dimethylformamide, wherein, nicotinoyl chloride and NH 2 -The mass ratio of MIL-101(Cr) is 1:1, pyridine and NH 2 -The mass ratio of MIL-101(Cr) is 0.5:1, N,N-dimethylformamide and NH 2 -The mass ratio of MIL-101(Cr) is 50:1, ultrasonic dispersion, reflux reaction for 6 hours, and centrifugation after cooling; the obtained solid is put into toluene, and bromobutane, toluene and NH 2 -The mass ratio of MIL-101(Cr) is 50:1, bromobutane and NH 2 -The mass ratio of MIL-101(Cr) is 1.5:1, ultrasonic dispersion, reflux reaction for 5 hours, centrifugation, drying to obtain F-NH 2 -MIL-101(Cr).
Embodiment 2
[0029] Catalyst preparation: NH 2 -MIL-101(Cr), nicotinoyl chloride and pyridine are added to N,N-dimethylformamide, wherein, nicotinoyl chloride and NH 2 -The mass ratio of MIL-101(Cr) is 1.5:1, pyridine and NH 2 -The mass ratio of MIL-101(Cr) is 0.8:1, N,N-dimethylformamide and NH 2 -The mass ratio of MIL-101(Cr) is 120:1, ultrasonic dispersion, reflux reaction for 12 hours, cooling and centrifugation; the obtained solid is put into toluene, and bromobutane, toluene and NH 2 -The mass ratio of MIL-101(Cr) is 120:1, bromobutane and NH 2 -The mass ratio of MIL-101(Cr) is 2:1, ultrasonic dispersion, reflux reaction for 8 hours, centrifugation, drying to obtain F-NH 2-MIL-101(Cr).
Embodiment 3
[0031] Catalyst preparation: NH 2 -MIL-101(Cr), nicotinoyl chloride and pyridine are added to N,N-dimethylformamide, wherein, nicotinoyl chloride and NH 2 -The mass ratio of MIL-101(Cr) is 2:1, pyridine and NH 2 -The mass ratio of MIL-101(Cr) is 1:1, N,N-dimethylformamide and NH 2 -The mass ratio of MIL-101(Cr) is 200:1, ultrasonic dispersion, reflux reaction for 24 hours, and centrifugation after cooling; the obtained solid is put into toluene, and bromobutane, toluene and NH 2 -The mass ratio of MIL-101(Cr) is 200:1, bromobutane and NH 2 -The mass ratio of MIL-101(Cr) is 3:1, ultrasonic dispersion, reflux reaction for 15 hours, centrifugation and drying to obtain F-NH 2 -MIL-101(Cr).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com