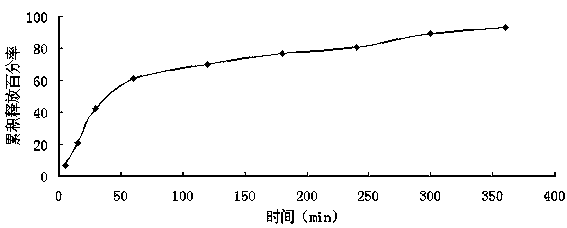
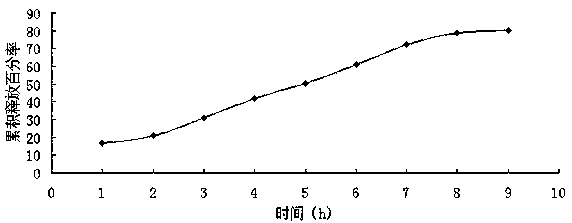
Buccal patch containing artificial bezoar and metronidazole and preparation method of buccal patch
A bezoar metronidazole and oral patch technology, applied in the field of pharmacy, can solve the problems of slow onset of action, large amount of raw and auxiliary materials, first-pass effect of the patient's liver, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of artificial bezoar metronidazole sustained-release double-layer oral patch
[0040] Drug-containing layer: Metronidazole 5.0g
[0041] Artificial bezoar 0.5g
[0042] β-CD33g
[0043] Carbomer 5g
[0044] HPMC5g
[0045] Dextrin 20.0g
[0046] Starch 9.5g
[0047] Aspartame 4g
[0048] Magnesium stearate 0.5g
[0049] Micropowder silica gel 0.5g
[0050] Protective layer: microcrystalline cellulose 15g
[0051] Lactose 5g
[0052] 70% ethanol 2g
Embodiment 2
[0053] Example 2: Preparation of artificial bezoar metronidazole sustained-release double-layer oral patch
[0054] Metronidazole 5.0g
[0055] Artificial bezoar 2.5g
[0056] HP-β-CD15g
[0057] Carbomer 10.0g
[0058] HPMC2.5g
[0059] Mannitol 25.5g
[0060] Mint powder 2g
[0061] Flavor 2g
[0062] Magnesium stearate 0.25g
[0064] Protective layer: starch 15g
[0065]Lactose 10g
[0066] 75% ethanol 2g
Embodiment 3
[0067] Example 3: Preparation of artificial bezoar metronidazole sustained-release double-layer oral patch
[0068] Drug-containing layer: Ornidazole 10g
[0069] Artificial bezoar 5g
[0070] β-CD80g
[0071] Carbomer 12g
[0072] HPMC2g
[0073] Mannitol 16.5g
[0074] borneol 2g
[0075] Mint powder 2g
[0076] Magnesium stearate 0.5g
[0077] Protective layer: microcrystalline cellulose 10g
[0078] Lactose 5g
[0079] 80% ethanol 2g
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com