Method for synthesizing C11 perfluoro-ketone
A technology of perfluoroketone and potassium fluoride, which is applied in the field of synthesizing C11 perfluoroketone, can solve the problems of low yield, high reaction temperature, and long reaction time, and achieve high reaction yield, simple operation, and fast reaction speed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
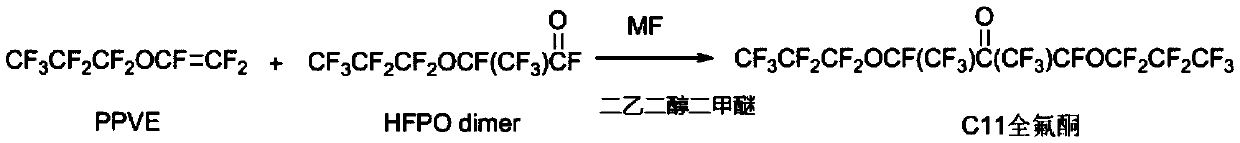
[0024] A method for synthesizing C11 perfluoroketone: Perfluoro-n-propyl vinyl ether (PPVE) 106g, hexafluoropropylene oxide dimer 144g, cesium fluoride 12g and diethylene glycol dimethyl ether 260mL are added to In a 1L reactor, raise the temperature to 95°C, stir and react for 2 hours, observe that the reaction pressure does not change, lower the temperature, open the reactor, separate the lower layer liquid and obtain 205g of product C11 perfluoroketone by conventional distillation with a yield of 86%.
[0025] Among them, the method for synthesizing perfluoro-n-propyl vinyl ether can be: using hexafluoropropylene oxide as a raw material to carry out self-dimerization (the actual reaction is two steps, and perfluoro-n-propyl vinyl ether is first isomerized to generate CF 3 CF 2 CFO, which can undergo a telomerization reaction with hexafluoropropylene oxide in one step, and the two-step reaction can be combined in the same reactor) to generate an acyl fluoride intermediate, w...
Embodiment 2
[0028] On the basis of Example 1, carry out scale-up production test: with perfluoro-n-propyl vinyl ether (PPVE) 1kg, hexafluoropropylene oxide dimer 1.5kg, cesium fluoride 120g and diglyme Add 2.6L into a 10L reactor, raise the temperature to 95°C, stir and react for 2 hours, observe that the reaction pressure does not change, lower the temperature, open the reactor, separate the lower layer liquid and distill to obtain the product C11 perfluoroketone 1.95kg, the yield is 87 %.
[0029] Facts have proved that using the method for synthesizing C11 perfluoroketones of the present invention, the product yield is greater than 80%, and the raw materials are easy to prepare, the reaction yield is improved, the operation is simple, easy to purify, and can be scaled up for production. Social value and economic value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com