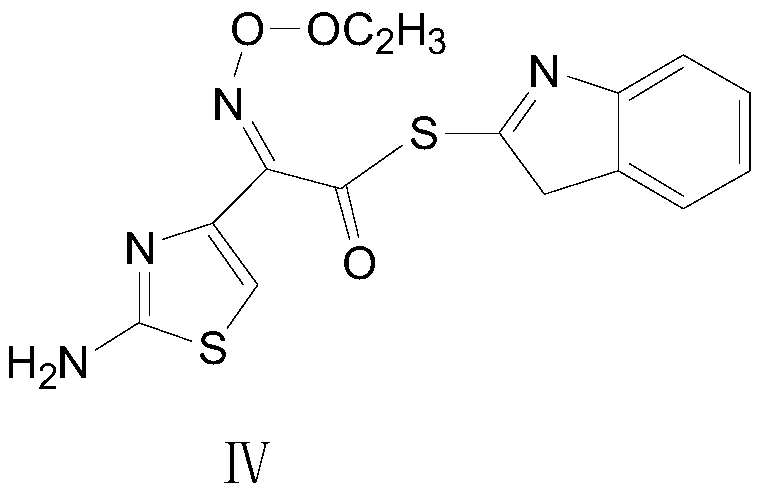
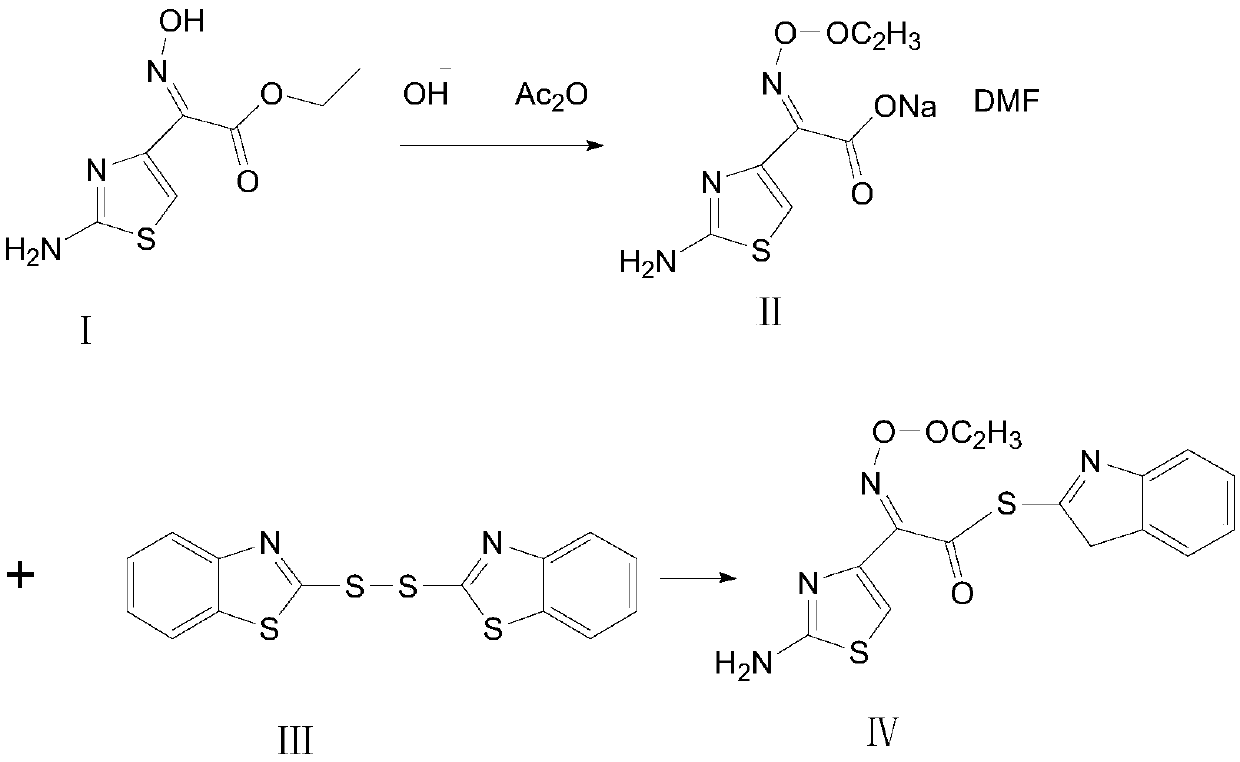
Synthetic method of cefdinir activated thioester
A technology of active thioesters and cefdinir, which is applied in the field of synthesis of cefdinir active thioesters, can solve the problems of difficult removal of crystal water, unstable product quality, and difficult control, etc., so that the use of less types of solvents is beneficial to large Large-scale production, the effect of easy pH control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] At room temperature, take a clean three-neck flask, add 50g (0.23mol) of ethyl demethylaminothiaxate, add 350ml of methanol, 50ml of DMF, stir for 30min, then add 19.5g (0.35mol) of potassium hydroxide, add 4.2g (0.23mol) of water , stirred, and reacted at 25°C for 2h;
[0040] Add 28.4g (0.28mol) of acetic anhydride dropwise to the reaction solution, react at 40°C for 1h, cool down to below 5°C, and filter with suction to obtain an adduct with the aprotic polar solvent DMF;
[0041] Add the adduct into the three-necked flask, add 300ml of dichloromethane, then add DM85g (0.26mol), add 50.2g (0.27mol) of triethyl phosphite dropwise at 18±2°C, take 1.5h, cool down to below 7°C, Suction filtration and vacuum drying at 40° C. for 4 hours gave 63 g of cefdinir active thioester with a molar yield of 71.8% and a purity of 99.05%.
Embodiment 2
[0043] At room temperature, take a clean three-neck flask, add 50g (0.23mol) of ethyl demethylaminothiaxate, add 545ml of ethanol, 55ml of DMAC, stir for 30min, then add 19.5g (0.35mol) of potassium hydroxide, add 4.2g (0.23mol) of water , stirred, and reacted at 25°C for 4h;
[0044] Add 28.4g (0.28mol) of acetic anhydride dropwise to the reaction solution, react at 40°C for 1 hour, cool down to below 5°C, and filter with suction to obtain the adduct with aprotic polar solvent DMAC;
[0045] Add the adduct into the three-necked flask, add 700ml of acetonitrile, then add DM100.4g (0.30mol), add 65.6g (0.40mol) of triethyl phosphite dropwise at 18±2°C, take 3h, cool down to below 7°C, pump Filtration, vacuum drying at 40°C for 4 hours, 63.5 g (0.40 mol) of cefdinir active thioester was obtained, the molar yield was 72.3%, and the purity was 99.03%.
Embodiment 3
[0047] At room temperature, take a clean three-necked flask, add 50g (0.23mol) of ethyl demethylthioxamate, add 200ml of isopropanol, 100ml of DMF, stir for 30min, then add 11.2g (0.28mol) of sodium hydroxide, add 8.3g (0.46mol ) water, stirred, and reacted at 25°C for 2h;
[0048] Add 20.4g (0.20mol) of acetic anhydride dropwise to the reaction solution, react at 42°C for 1h, cool down to below 5°C, and filter with suction to obtain the adduct with the aprotic polar solvent DMF;
[0049] Add the adduct into the three-necked flask, add 600ml of toluene, then add DM100.4g (0.30mol), add 65.6g (0.40mol) of triethyl phosphite dropwise at 18±2°C, take 2h, cool down to below 7°C, pump Filtration, vacuum drying at 38° C. for 4 hours, to obtain 63.5 g of cefdinir active thioester, with a molar yield of 72.3% and a purity of 99.10%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com