Nitrogen-doped graphene-ferronickel hydrotalcite difunctional oxygen catalyst and preparation method and application thereof
A nitrogen-doped graphene and oxygen catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., to achieve the effects of easy purchase and preparation, increased specific surface area, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (a) Preparation of GO / LDH complex
[0035] Disperse a certain amount of GO in ethylene glycol to make the concentration 1.0mg / mL, ultrasonically disperse for 1 hour, and centrifuge at 3000rpm for 10min to remove unstripped GO to obtain a stripped GO dispersion. Take 20mL of the dispersion and press 3: Add nickel chloride hexahydrate and ferric chloride hexahydrate to it at a molar ratio of 1, so that the total concentration of metal ions is 0.04mol / L, stir to make it dissolve completely, then slowly add 0.6g sodium dodecylsulfonate, stir Under the conditions, make it completely dissolved, then drop into 10mL ethylene glycol solution containing 0.16g NaOH at a constant speed, move the mixed solution into the reaction kettle, react at 160°C for 24h, and wash the reaction solution three times with deionized water and ethanol after centrifugation , which is the GO / LDH complex after drying;
[0036] (b)C 3 N 4 Preparation of nanosheets
[0037] Calcining 0.5g melamine at...
Embodiment 2
[0041] (a) Preparation of GO / LDH complex
[0042] Prepared according to the method and conditions of step (a) in Example 1;
[0043] (b)C 3 N 4 Preparation of nanosheets
[0044] Prepared according to the method and conditions of step (b) in Example 1;
[0045] (c) Preparation of NG / LDH oxygen catalyst
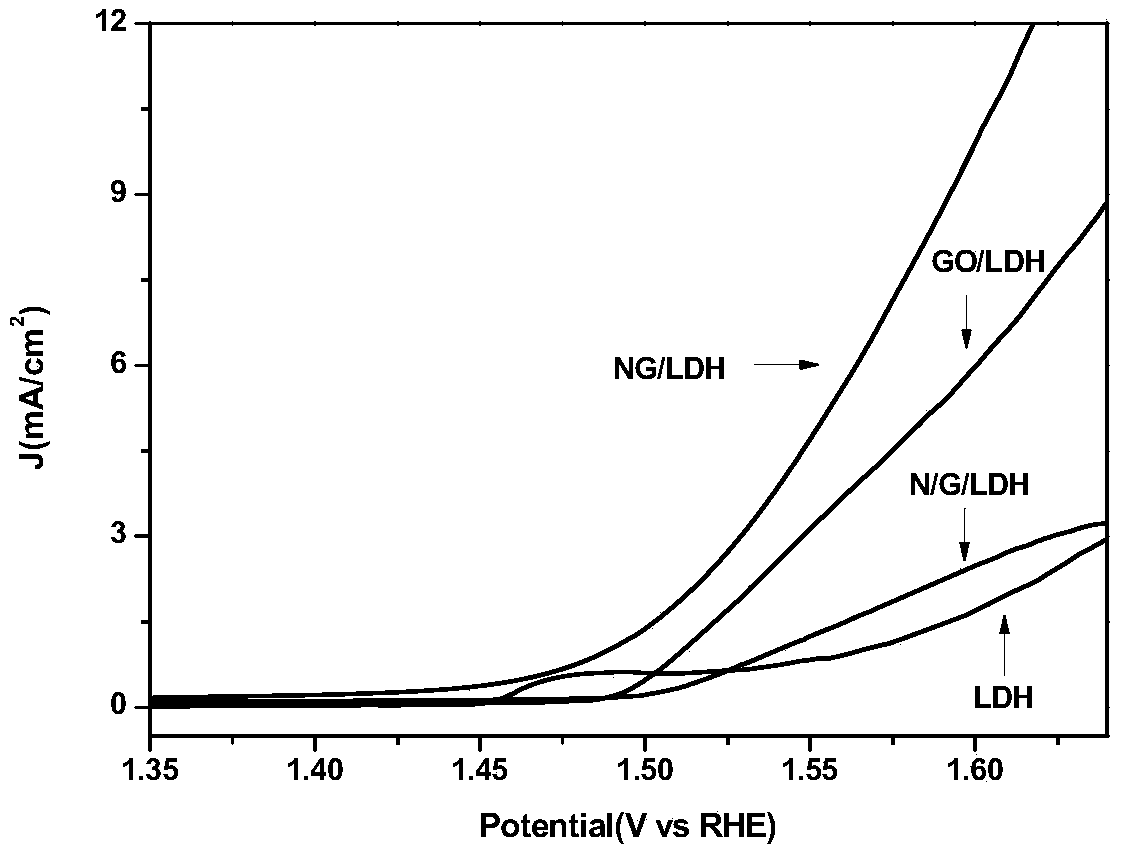
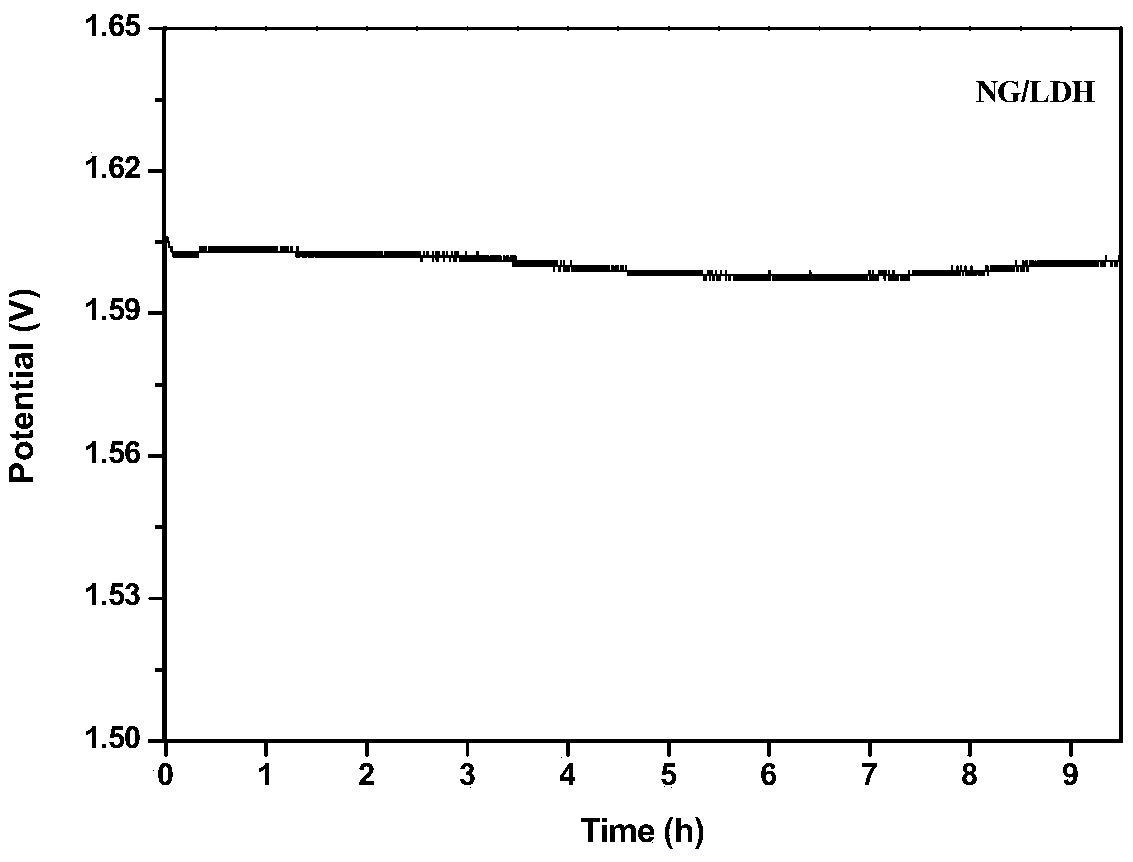
[0046] Disperse the GO / LDH complex obtained in step (a) in an aqueous solution to a concentration of 2 mg / ml, pipette 50 ml of the solution, and add 30 mL of 0.75 mg / mL of C 3 N 4 The aqueous solution of nanosheets was mixed and moved to the reaction kettle, and reacted at 180°C for 20 hours. The obtained solid sample was centrifuged, washed with deionized water and ethanol three times, and dried to obtain the bifunctional oxygen catalyst NG / LDH. Its average particle size is 296nm and its specific surface area is 186.51m 2 / g.
Embodiment 3
[0048] (a) Preparation of GO / LDH complex
[0049] Prepared according to the method and conditions of step (a) in Example 1;
[0050] (b)C 3 N 4 Preparation of nanosheets
[0051] Prepared according to the method and conditions of step (b) in Example 1;
[0052] (c) Preparation of NG / LDH oxygen catalyst
[0053] Disperse the GO / LDH complex obtained in step (a) in an aqueous solution to a concentration of 2mg / ml, pipette 50ml of the solution, and add 30mL of 0.50mg / mL of C 3 N 4 The aqueous solution of nanosheets was mixed and moved to the reaction kettle, and reacted at 180°C for 20 hours. The obtained solid sample was centrifuged, washed with deionized water and ethanol three times, and dried to obtain the bifunctional oxygen catalyst NG / LDH. Its average particle size is 318nm and its specific surface area is 208.63m 2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com