Palladium catalyzed synthesis method of 2-(2 '-hydroxy phenyl) benzoxazole
A technology of hydroxyphenyl and benzoxazole, which is applied in the field of synthesis of 2-benzoxazole, can solve the problems of low catalytic reaction efficiency, many by-products, large usage, etc., and achieves easy implementation, few by-products, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
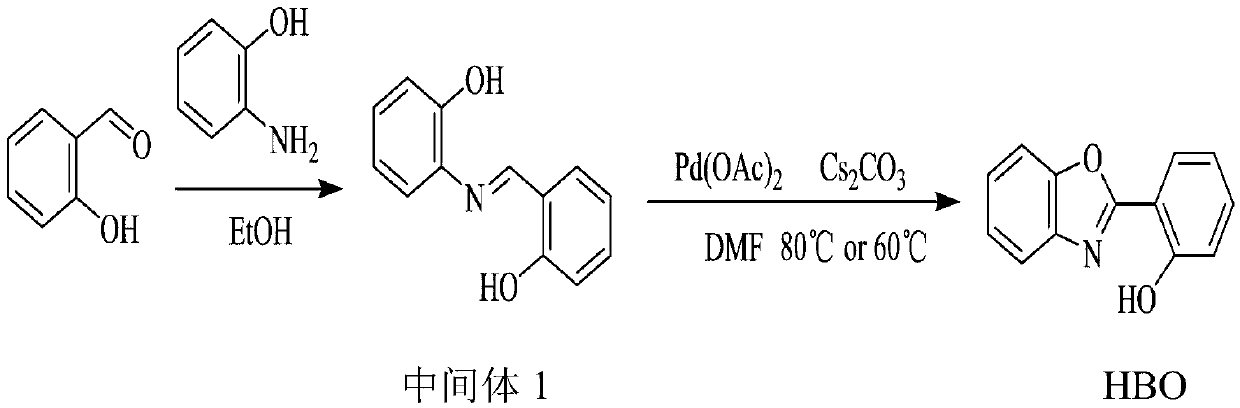
[0031] This example uses Pd(OAc) 2 and Cs 2 CO 3 , DMF and a warming environment to synthesize HBO.
[0032] figure 1 The preparation route for this example is shown.
[0033] Step A, condensation reaction
[0034] Salicylaldehyde (5mg, 0.04mmol) and 2-aminophenol (9mg, 0.08mmol) were added to 15mL of anhydrous EtOH, and refluxed for 5 hours; after cooling, the solution was filtered, and the precipitate was recrystallized with anhydrous EtOH to obtain Red needle crystal Schiff base intermediate 1.
[0035] The structural formula of the intermediate 1 is:
[0036] Step B, oxidation ring formation reaction
[0037] Intermediate 1 (43mg, 0.2mmol), Pd(OAc) 2 (2.3mg, 0.01mmol) and Cs 2 CO 3 (130mg, 0.4mmol) was dissolved in 10mL of DMF (dimethylformamide) solution, stirred at room temperature for 5 minutes, then heated to 80°C, and at the same time, oxygen was continuously introduced below the liquid surface;
[0038] Step C, using TLC method (thin layer chromatography...
Embodiment 2
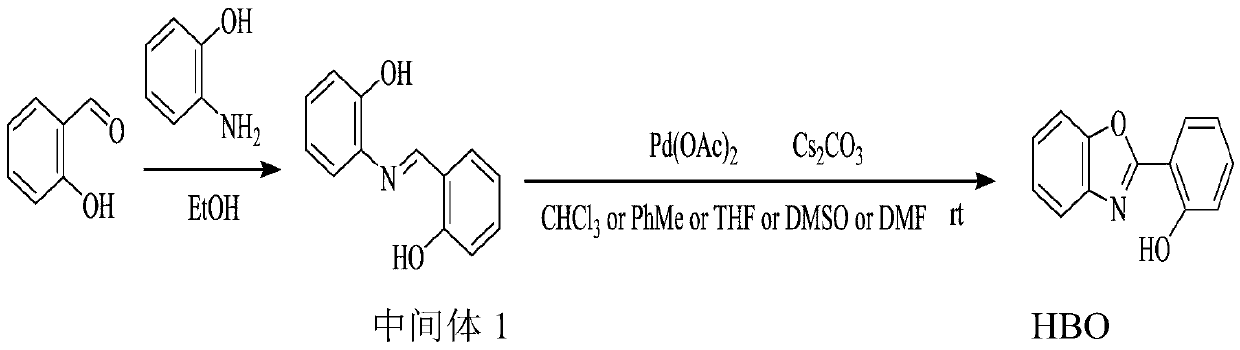
[0044] This example uses Pd(OAc) 2 and Cs 2 CO 3 , DMF and a warming environment to synthesize HBO.
[0045] figure 1 The preparation route for this example is shown.
[0046] Step A, condensation reaction
[0047] This step is the same as the best embodiment 1.
[0048] Salicylaldehyde (5mg, 0.04mmol) and 2-aminophenol (9mg, 0.08mmol) were added to 15mL of anhydrous EtOH, and refluxed for 5 hours; after cooling, the solution was filtered, and the precipitate was recrystallized with anhydrous EtOH to obtain Red needle crystal Schiff base intermediate 1.
[0049] The structural formula of the intermediate 1 is:
[0050] Step B, oxidation ring formation reaction
[0051] Intermediate 1 (43mg, 0.2mmol), Pd(OAc) 2 (2.3mg, 0.01mmol) and Cs 2 CO 3 (130mg, 0.4mmol) was dissolved in 10mL of DMF (dimethylformamide) solution, stirred at room temperature for 5 minutes, then heated to 60°C for reaction, and at the same time, oxygen was continuously introduced below the liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com