CAPRI cell and preparation method thereof
A cell and nuclear cell technology, applied in the field of CAPRI cells and their preparation, can solve the problems of doubling the probability of cell culture contamination, production safety and clinical cooperation, tumor killing immune response and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
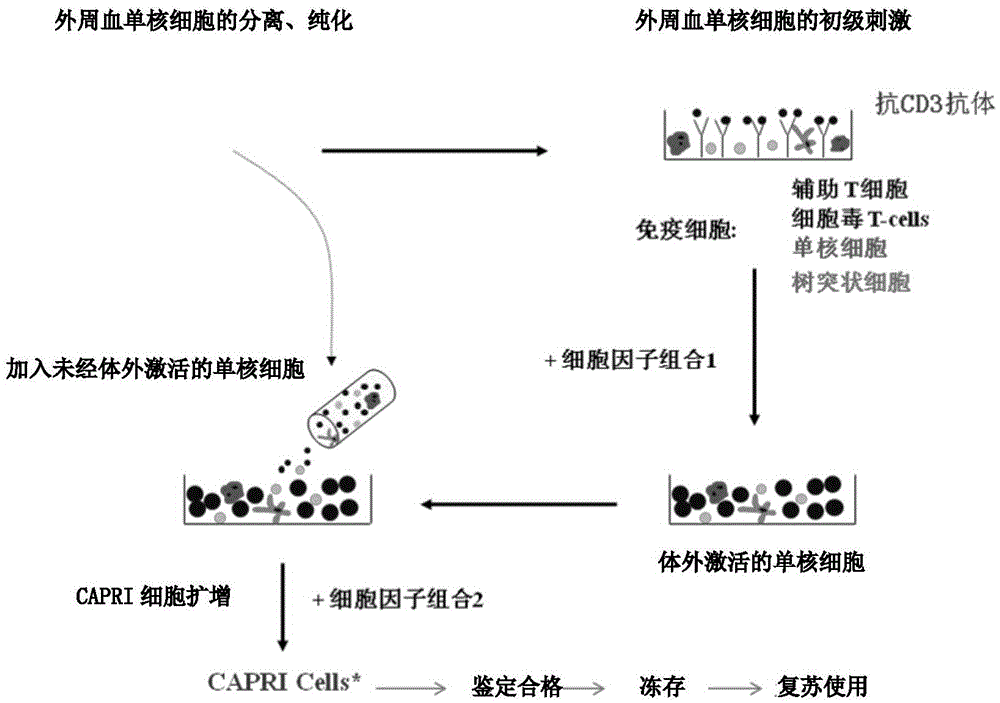
[0060] Example 1 Preparation of Cascade Cytokine-Induced and Activated Tumor-Specific Killer T Lymphocytes (CAPRI Cells)
[0061] 1. Peripheral Blood Mononuclear Cell Collection
[0062] 1.1 Method 1: Human peripheral blood collection: use heparin anticoagulation, manually collect blood 120ml-400ml, after separation and purification, the number of monocytes is about 1.5×10 8 -5.0×10 8 .
[0063] 1.2 Method 2: Obtain peripheral blood mononuclear cells from the patient through leukapheresis technology. The volume of apheresis is 150-500ml. 8 -5.0×10 8 .
[0064] 2. Preparation of reagents and solutions
[0065] 2.1 Preparation of coating solution
[0066] 2.1.1 Prepare 1× Phosphate Buffer Saline (PBS) with pH7.0-7.4
[0067] 2.2.2 Adjust the pH of the above PBS buffer to 8.6 with 1N sodium hydroxide solution
[0068] 2.2.3 Filter with 0.22μm sterile filter
[0069] 2.2.4 Add 0.2 ml of mouse anti-human CD3 monoclonal antibody (OKT3) at a concentration of 1 mg / ml to 100 m...
Embodiment 2
[0117] Example 2 In vitro experiment of CAPRI cells on autologous breast cancer cell cytotoxicity
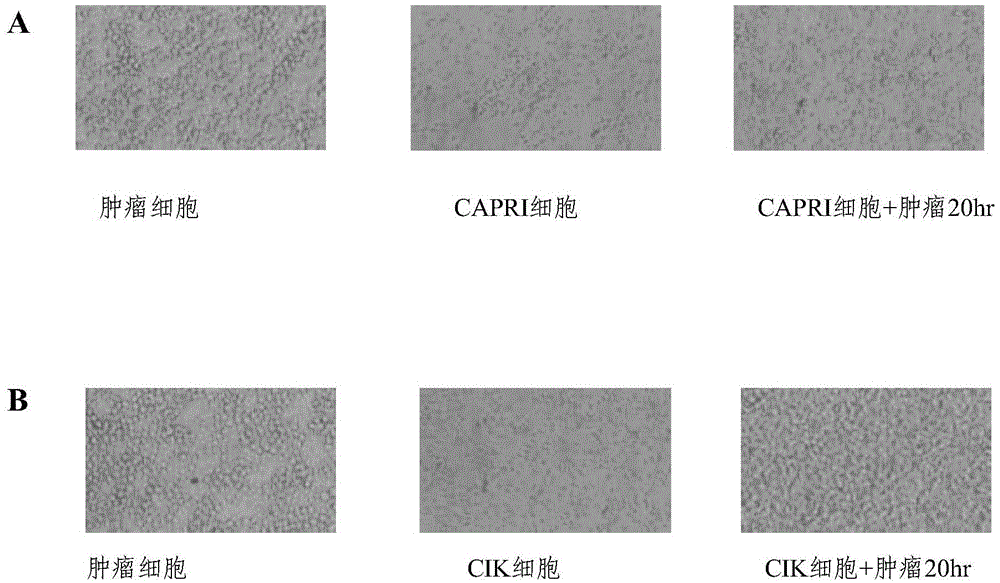
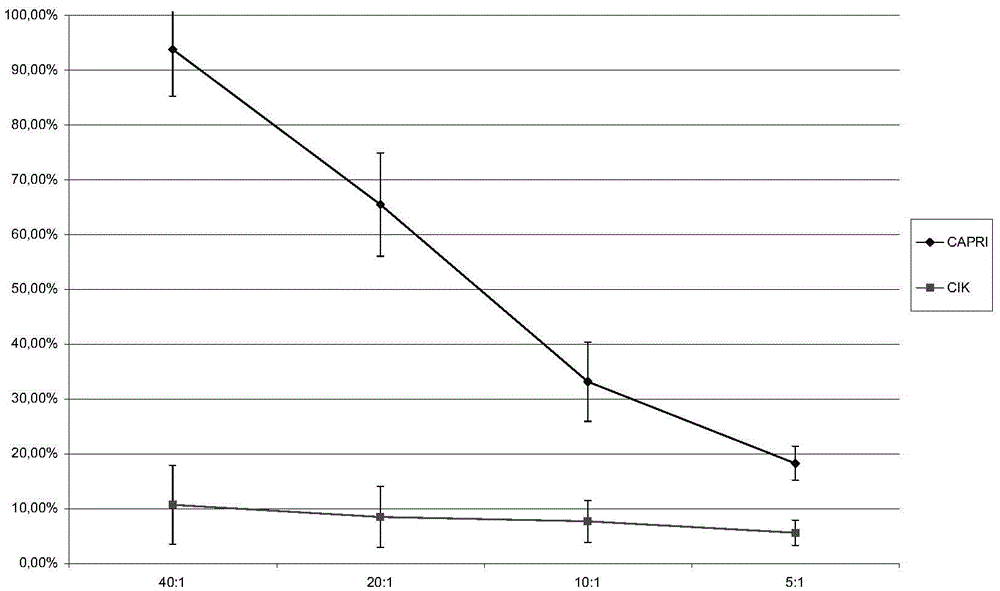
[0118] The method of Example 1 and the CIK technology were used to culture the patient's own CAPRI cells and CIK cells respectively, and the patient's own breast cancer cell line cells were used as target cells (marked with Cr51) for cytotoxicity experiments. The killing rate was measured by observation under a microscope and the amount of Cr51 radioactive release. The results are shown in figure 2 and image 3 .
[0119] in, figure 2 A is observation under the microscope that after the autologous tumor cells were co-incubated with CAPRI cells and CIK cells for 20 hours, the number of residual tumors in the CAPRI cell group was significantly less than that in the CIK cell group, which proves that CAPRI cells have obvious killing and inhibitory effects on autologous tumor cells in vitro ; but under the same conditions, the effect of CIK cells was not obvious ( figure 2 B)....
Embodiment 3
[0120] In vivo test of embodiment 3CAPRI cell activity
[0121] 1×10 autologous tumor cells from patients with colorectal cancer 5 Each was subcutaneously injected into nude mice at the age of 6-8 weeks. At the same time, the patient's peripheral blood mononuclear cells were used to culture CAPRI cells according to the method of Example 1. CIK cells were prepared by CIK technology, and the tail vein injection containing 1 × 10 6 0.1ml of normal saline for each cell, administered continuously for one week. After 21 days or when the mice died, the tumor volume was measured and the survival time of the mice was recorded (observed for 45 days in total). The statistical analysis results are shown in Table 2, Figure 4-Figure 5 .
[0122] Table 2 Comparison of the largest tumor diameter in nude mice
[0123] CAPRI cell group CIK cell group 3.3mm 7.1mm 2.5mm 6.3mm 3.7mm 6.8mm 2.9mm 6.9mm 3.4mm 7.5mm 2.7mm 5.9mm 3.083±0.187...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com