Method for preparing lithium iron phosphate by hydrothermal method and lithium iron phosphate prepared by method
A lithium iron phosphate and hydrothermal technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of complex operation, affecting the pH value of the reaction system, lengthy process, etc., and achieve the goal of inhibiting particle growth, simple preparation method, The effect of high discharge specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In this example, LiOH·H 2 O, H 3 PO 4 and FeSO 4 ·7H 2 O is used as raw material, and the molar ratio of Li:Fe:P is 3:1:1, and the improved hydrothermal method is used to prepare 0.3mol LiFePO 4 . details as follows:
[0037] a. LiOH·H 2 O is prepared into a 3mol / L lithium source solution, and the H 3 PO 4 Prepare a 1.5mol / L phosphorus source solution, slowly add the phosphorus source solution into the lithium source solution in proportion to make a mixed solution A;
[0038] b. FeSO 4 ·7H 2 O is prepared into a 1mol / L iron source solution, and added to the iron source solution to theoretically generate LiFePO 4 20wt.% of the total weight of starch, mixed to make mixed solution B;
[0039] c. Slowly add the mixed solution B into the mixed solution A under mechanical stirring to form a reaction system solution, and transfer the reaction system solution to a 1L reactor for reaction. After the reaction is completed, filter to obtain LiFePO 4 product.
[0040]...
Embodiment 2
[0043] This example is the same as the method of Example 1, wherein the amount of starch added in the iron source solution is theoretically generated LiFePO 4 10wt.% of the total weight, all the other components and consumption are the same.
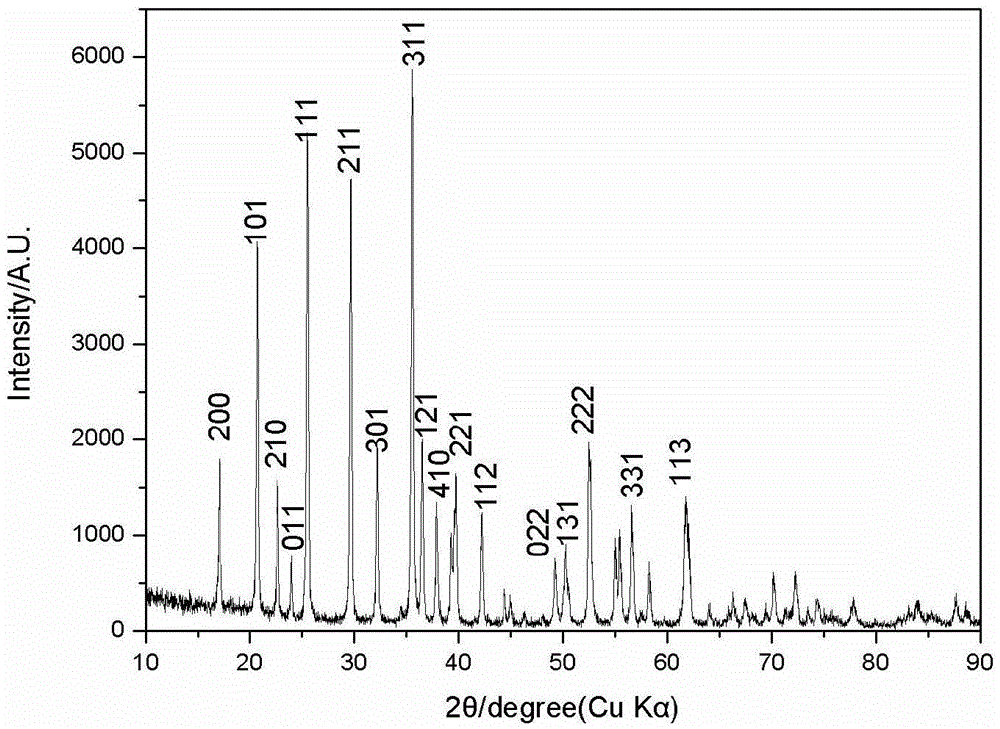
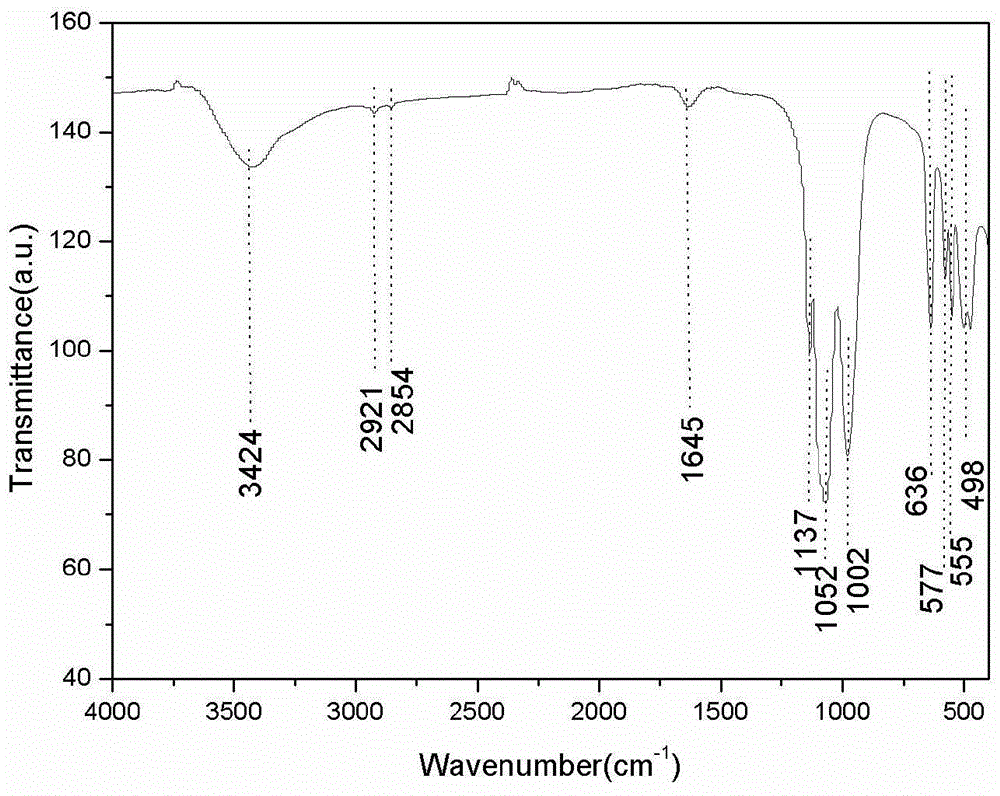
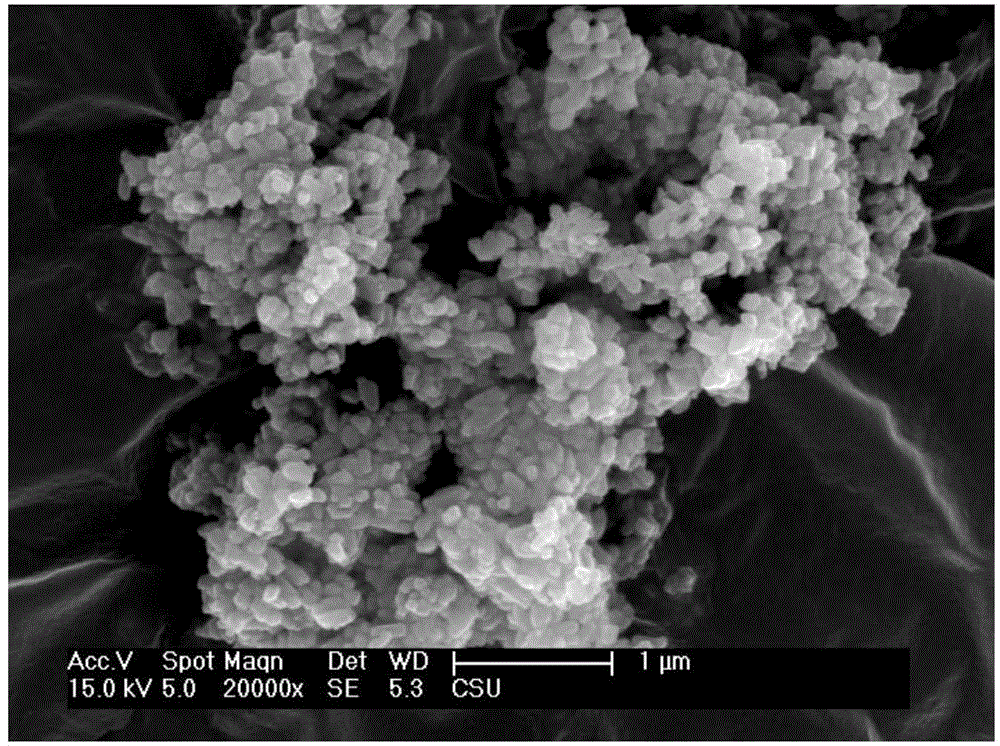
[0044] For the LiFePO prepared in this example 4 Product is carried out scanning electron microscope observation, it can be seen that the LiFePO prepared in this example 4 The product is prismatic, and as Figure 4 As shown, the particle size is about 1 μm in length and 200 nm in thickness, indicating that the main reason for the change in product morphology is the change in starch content. It can be seen that starch plays a role in inhibiting particle size and controlling the formation of morphology in the system reaction. The high-resolution transmission electron microscope results showed that there were disorderly arranged small particles of lithium iron phosphate on the surface of the product, indicating that the starch acted as a ...
Embodiment 3
[0046] This example is the same as the method of Example 1, wherein the amount of starch added in the iron source solution is theoretically generated LiFePO 4 The 30wt.% of total weight, all the other components and consumption are the same.
[0047] For the LiFePO prepared in this example 4 Product is carried out scanning electron microscope observation, it can be seen that the LiFePO prepared in this example 4 The product is in the shape of a rib, and the particle size is about 1 μm in length and 200 nm in thickness; LiFePO 4 The product is similar to Example 2, and there are small particles of lithium iron phosphate disorderly arranged on the surface of the particles, as shown in the scanning electron microscope Figure 5 As shown, it can be seen that with the change of starch content in the reaction system, there is an obvious trend in the control of particle size and shape. At 20wt.%, spherical lithium iron phosphate products can be prepared, and diamond-shaped granula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com