Drinking food containing phosphatidylserine, and manufacturing method therefor
A technology of phosphatidylserine and phosphatidylserine, which is applied in the field of food and drink containing phosphatidylserine and its manufacture, can solve the problems of inability to add in large quantities, change in taste, and cannot withstand sufficient practical application, and achieves improved dispersion. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
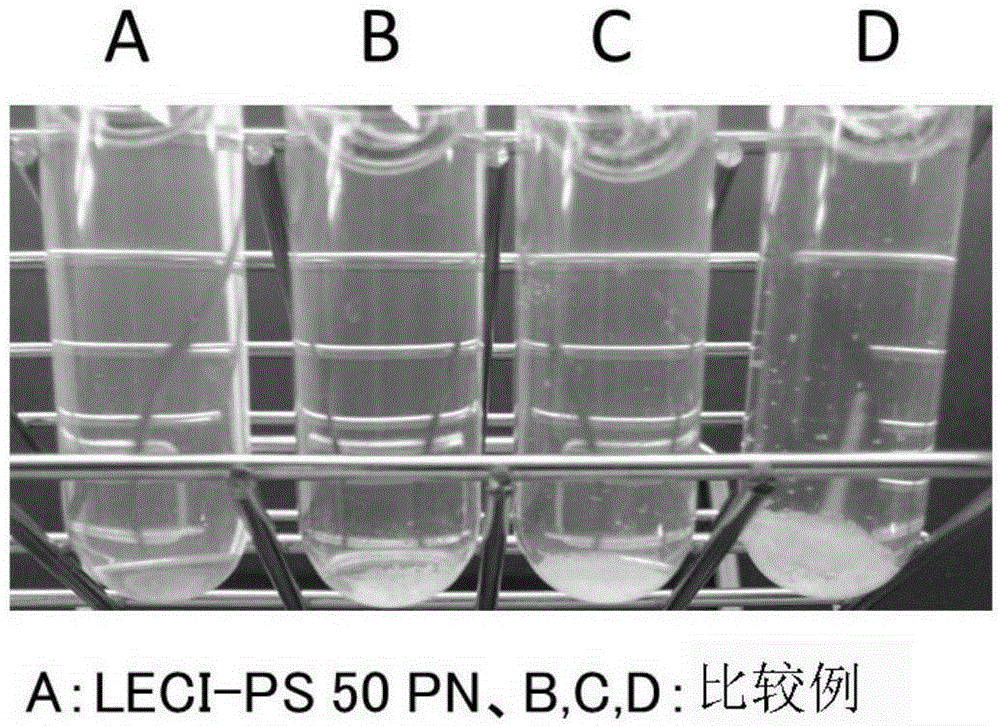
[0052] 20 mg of PS described in LECI-PS50PN and Comparative Examples (B: Lipogen, C: Doosan, D: Xian) were added to 20 g of water or milk, and stirred at room temperature for 15 minutes. After the stirring was completed, 5 ml of the PS dispersion liquid was taken, transferred to a test tube, and the generation of a precipitate was confirmed visually. figure 1 Indicates the state of precipitation when dissolved in water, figure 2 The state of precipitation when dissolved in milk is shown. Depend on figure 1 and figure 2 It was found that the product of the present invention to which LEC1-PS50PN was added had the least amount of PS precipitation when added to water and milk.
Embodiment 2
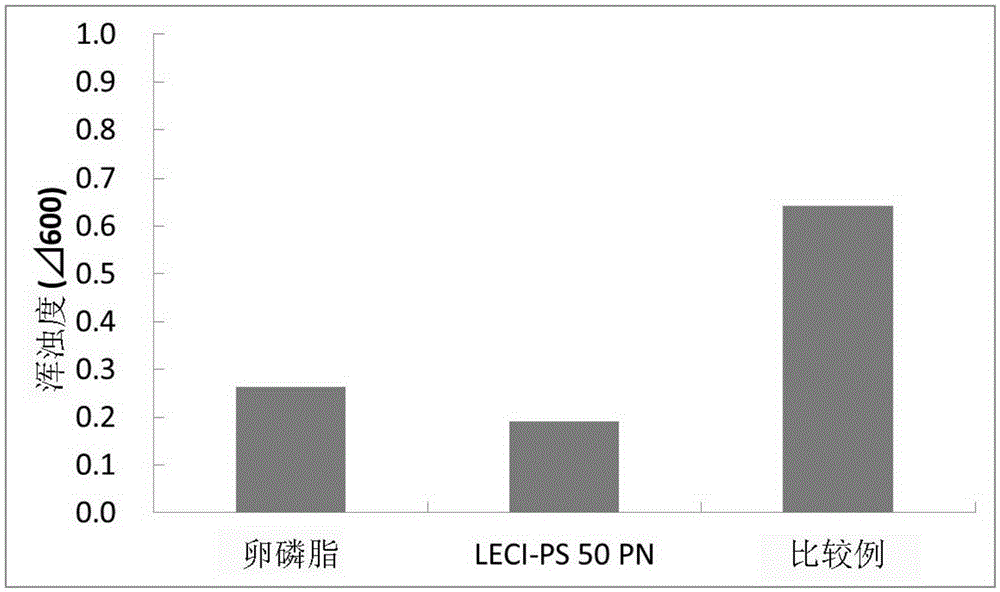
[0054] Regarding the clarity, the solution state was compared using powder lecithin (soybean lecithin), LECI-PS50PN, and a comparative example (manufactured by Doosan Corporation). Turbidity was measured by preparing powdered lecithin (soybean lecithin), LECI-PS50PN, and a 1% aqueous solution of PS described in Comparative Example (manufactured by Doosan Co., Ltd.), and measuring the absorbance at 600 nm to evaluate clarity ( image 3 ). As a result, the product of the present invention to which LECI-PS50PN was added showed a turbidity of about one-third or less compared with the conventional product (comparative example). In a narrow sense, "lecithin" means phosphatidylcholine, which is one type of phospholipid, and in a broad sense, it means a mixture mainly composed of phospholipids such as phosphatidylcholine and phosphatidylethanolamine.
Embodiment 3
[0056] In addition, for the PS described in LECI-PS50PN and Comparative Example (manufactured by Doosan Corporation), the remaining amount of PS after heat-treating a 0.5% aqueous solution at 90° C. for 4 minutes was measured ( Figure 4 ). Regarding the remaining amount of PS, a solution in which 100 μl of a 0.5% aqueous solution was dissolved in chloroform:methanol=2:1 was used as an analysis sample, and analyzed by HPLC. The peak areas before the heat treatment and after the heat treatment were calculated respectively, and the remaining ratio was calculated by setting the before heat treatment as 100. As a result, the product of the present invention to which LECI-PS50PN was added showed little change in the remaining PS amount after heating, but in the comparative example (manufactured by Doosan Co.), the remaining PS amount decreased by about 15%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com