Preparation method and application of fluorescent gold nanoparticles
A technology of fluorescent gold nanoparticles and particles, applied in the directions of nanotechnology, nanotechnology, fluorescence/phosphorescence, etc., can solve the problems of short fluorescence quantum yield and fluorescence lifetime of gold nanoparticles, limited use range, strong irritation to mucous membranes, etc. Effects of biocompatibility, high sensitivity and selectivity, simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
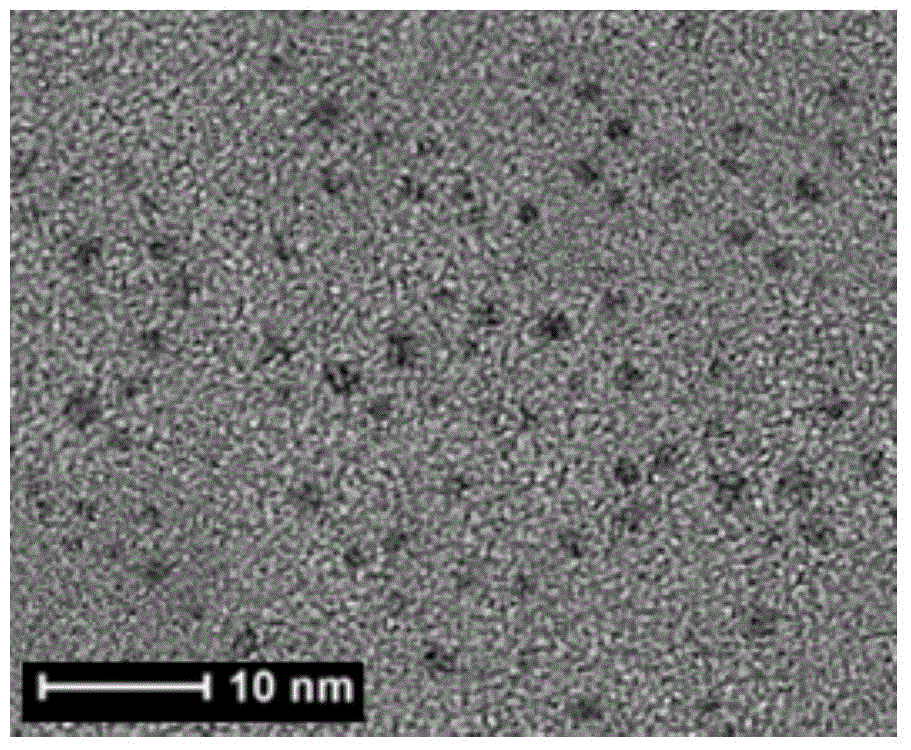
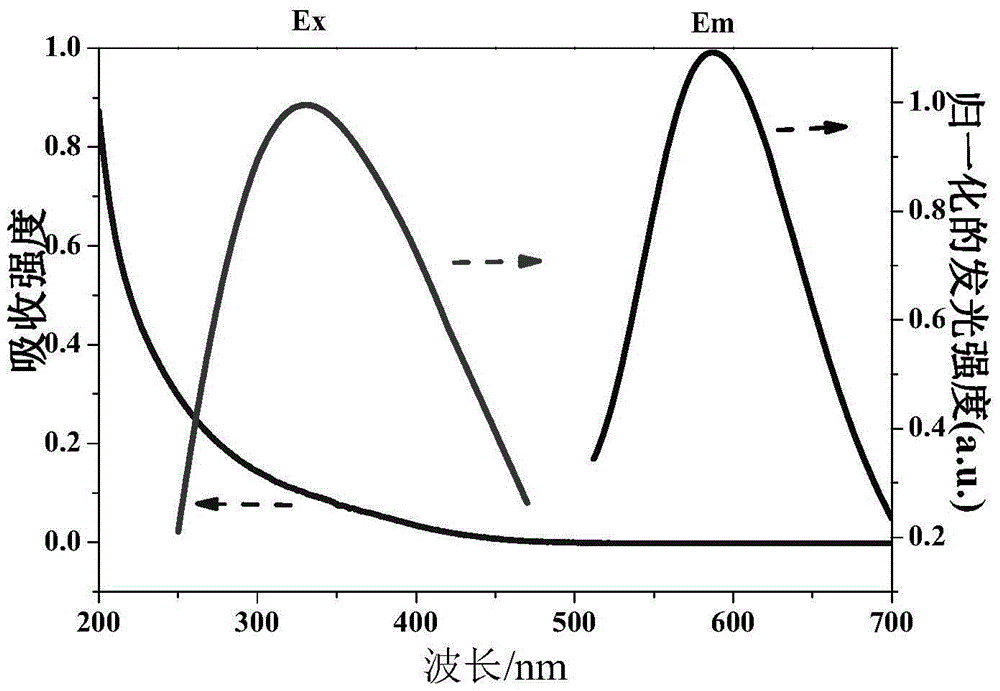
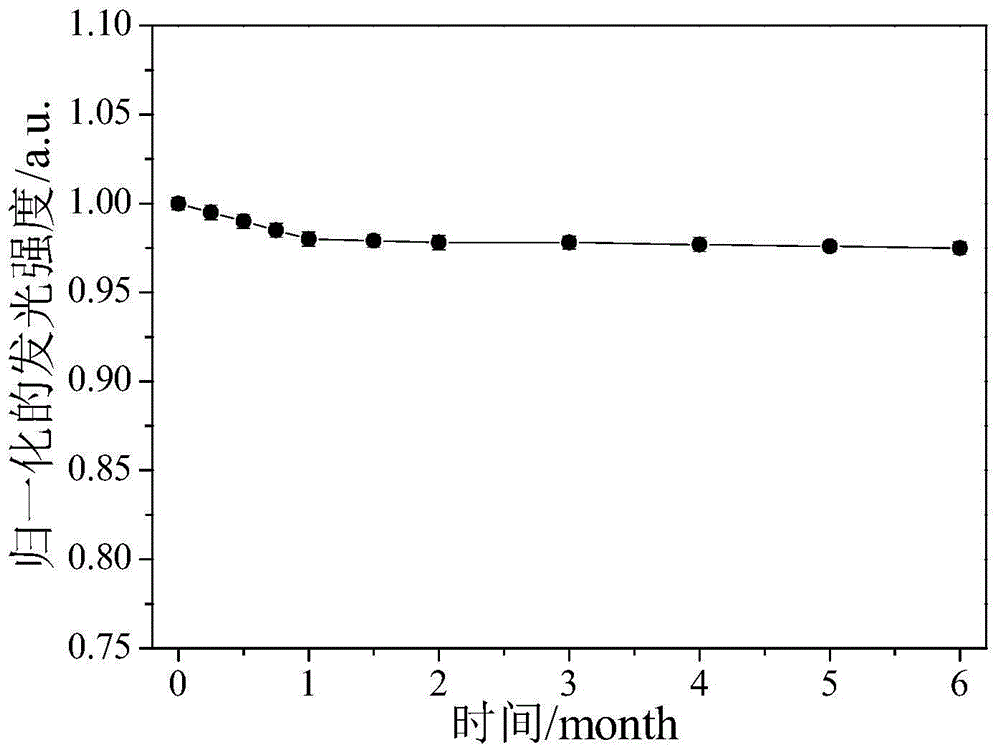
[0019] Mix 0.2mL 100mmol / L N-acetyl-L-cysteine aqueous solution, 1.0mL 100mmol / L chloroauric acid aqueous solution and 5.0mL methanol / glacial acetic acid solution with a volume ratio of 6:1, stir for 60min, and then add to the above 3.8mL of ultrapure water was added to the mixture, stirred and heated at 90°C, refluxed for 48 hours, taken out after cooling, dialyzed and dried to obtain fluorescent gold nanoparticles. The fluorescence emission peak of the gold nanoparticles is around 550nm, and the Stocks shift is 150nm. Under ultraviolet light, when observed with a black background, it presents strong yellow-orange fluorescence, with a quantum yield of 1.0% and a fluorescence lifetime of 0.7μs.
Embodiment 2
[0021] Mix 0.4mL 100mmol / L N-acetyl-L-cysteine aqueous solution, 1.0mL 100mmol / L chloroauric acid aqueous solution and 4.0mL methanol / glacial acetic acid solution with a volume ratio of 6:1, stir for 50min, and then add to the above 4.6mL of ultrapure water was added to the mixture, stirred and heated at 80°C, refluxed for 36h, taken out after cooling, dialyzed and dried to obtain fluorescent gold nanoparticles. The fluorescence emission peak of the gold nanoparticles is around 560nm, and the Stocks shift is 170nm. Under ultraviolet light, when observed with a black background, it presents strong yellow-orange fluorescence, with a quantum yield of 2.5% and a fluorescence lifetime of 1.5μs.
Embodiment 3
[0023] Mix 0.4mL 100mmol / L N-acetyl-L-cysteine aqueous solution, 0.6mL 100mmol / L chloroauric acid aqueous solution and 3.0mL methanol / glacial acetic acid solution with a volume ratio of 6:1, stir for 40min, and then Add 6.0mL of ultrapure water to the above mixed solution, continue to stir and reflux at a heating temperature of 70° C. for 36 hours, take it out after cooling, dialyze and dry to obtain fluorescent gold nanoparticles. The fluorescence emission peak of the gold nanoparticles is around 590nm, and the Stocks shift is 250nm. Under ultraviolet light, when observed with a black background, it presents strong orange fluorescence with a quantum yield of 6.0% and a fluorescence lifetime of 6.3μs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com